7.8: Acid–Base and Gas Evolution Reactions
- Page ID
- 98034
- Identify when a reaction will evolve a gas.
Neutralization Reactions
Acids and bases react chemically with each other to form salts. A salt is a general chemical term for any ionic compound formed from an acid and a base. In reactions where the acid is a hydrogen-ion-containing compound and the base is a hydroxide-ion-containing compound, water is also a product. The general reaction is as follows:
\[\text{acid + base} → \text{water + salt} \nonumber \]
The reaction of acid and base to make water and a salt is called neutralization. Like any chemical equation, a neutralization chemical equation must be properly balanced. For example, the neutralization reaction between sodium hydroxide and hydrochloric acid is as follows:
\[\ce{NaOH (aq) + HCl (aq) \rightarrow NaCl (aq) + H_2O (ℓ)} \label{Eq2} \]
with coefficients all understood to be one. The neutralization reaction between sodium hydroxide and sulfuric acid is as follows:
\[\ce{2NaOH (aq) + H_2SO_4 (aq) \rightarrow Na_2SO_4(aq) + 2H_2O (ℓ)} \label{Eq3} \]
Nitric acid (HNO3(aq)) can be neutralized by calcium hydroxide (Ca(OH)2(aq)). Write a balanced chemical equation for the reaction between these two compounds and identify the salt that it produces.
Solution
| Steps | Explanation | Equation |
|---|---|---|
| Write the unbalanced equation. | This is a double displacement reaction, so the cations and anions swap to create new products. | Ca(OH)2(aq) + HNO3(aq) → Ca(NO3)2(aq) + H2O(ℓ) |
| Balance the equation. | Because there are two OH− ions in the formula for Ca(OH)2, we need two moles of HNO3 to provide H+ ions | Ca(OH)2(aq) + 2HNO3(aq) → Ca(NO3)2(aq) + 2H2O(ℓ) |
| Additional step: identify the salt. | The salt formed is calcium nitrate. |
Hydrocyanic acid (\(\ce{HCN(aq)}\)) can be neutralized by potassium hydroxide (\(\ce{KOH(aq)}\)). Write a balanced chemical equation for the reaction between these two compounds and identify the salt that it produces.
\[\ce{KOH (aq) + HCN(aq) → KCN (aq) + H2O(ℓ)} \nonumber \]
Gas Evolving Reactions
A gas evolution reaction is a chemical process that produces a gas, such as oxygen or carbon dioxide. In the following examples, an acid reacts with a carbonate, producing salt, carbon dioxide, and water, respectively. For example, nitric acid reacts with sodium carbonate to form sodium nitrate, carbon dioxide, and water (Table \(\PageIndex{1}\)):
\[\ce{2HNO3(aq)+Na2CO3(aq)→2NaNO3(aq)+CO2(g)+H2O(l)} \nonumber \]
Sulfuric acid reacts with calcium carbonate to form calcium sulfate, carbon dioxide, and water:
\[\ce{H2SO4(aq) + CaCO3(aq) → CaSO4(aq) + CO2(g)+H2O(l)} \nonumber \]
Hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water:
\[\ce{2HCl(aq) + CaCO3(aq) → CaCl2(aq) + CO2(g) + H2O(l)} \nonumber \]
Figure \(\PageIndex{1}\) demonstrates this type of reaction:
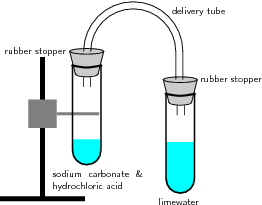
In this reaction setup, lime water, a dilute calcium hydroxide (\(Ca(OH)_2\)) solution, is poured into one of the test tubes and sealed with a stopper. A small amount of hydrochloric acid is carefully poured into the remaining test tube. A small amount of sodium carbonate is added to the acid, and the tube is sealed with a rubber stopper. The two tubes are connected. As a result of the acid-carbonate reaction, carbon dioxide is produced and the lime water turns milky.
| Reactant Type | Intermediate Product | Gas Evolved | Example |
|---|---|---|---|
| sulfide | none | \(\ce{H2S}\) | \(\ce{2HCl(aq) + K2S \rightarrow H2S (g) + 2KCl (aq)}\) |
| carbonates and bicarbonates | \(\ce{H2CO3}\) | \(\ce{CO2}\) | \(\ce{2HCl(aq) + K2CO2 \rightarrow H2O (l) + CO2(g) + 2KCl (aq)}\) |
| sulfites and bisulfites | \(\ce{H2SO3}\) | \(\ce{SO2}\) | \(\ce{2HCl(aq) + K2SO2 \rightarrow H2O (l) + SO2(g) + 2KCl (aq)}\) |
| ammonia | \(\ce{NH4OH}\) | \(\ce{NH3}\) | \(\ce{NH4Cl(aq) + KOH \rightarrow H2O (l) + NH3(g) + 2KCl (aq)}\) |
The gas-evolving experiment lime water is illustrated in the following video:
Video \(\PageIndex{1}\): Carbon Dioxide (\(CO_2\)) & Limewater (Chemical Reaction). As the reaction proceeds, the limewater on the turns from clear to milky; this is due to the \(CO_2(g)\) reacting with the aqueous calcium hydroxide to form calcium carbonate, which is only slightly soluble in water.
When this experiment is repeated with nitric or sulfuric acid instead of \(HCl\), it yields the same results: the clear limewater turns milky, indicating the production of carbon dioxide. Another method to chemically generate gas is the oxidation of metals in acidic solutions. This reaction will yield a metal salt and hydrogen gas.
\[\ce{2HCl (aq) + Zn(s) \rightarrow ZnCl_2 (aq) + H_2 (g)} \nonumber \]
Here, hydrochloric acid oxidizes zinc to produce an aqueous metal salt and hydrogen gas bubbles. Recall that oxidation refers to a loss of electrons, and reduction refers to the gain of electrons. In the above redox reaction, neutral zinc is oxidized to \(Zn^{2+}\), and the acid, \(H^+\), is reduced to \(H_2(g)\). The oxidation of metals by strong acids is another common example of a gas evolution reaction.
Contributors & Affiliations
Boundless (www.boundless.com)
Wikipedia (CC-BY-SA-3.0)
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).

