Carbonates
- Page ID
- 542
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Carbonate is a polyatomic anion with the formula \(CO_3^{2-}\) and has a trigonal planar molecular structure which consists of a carbon atom surrounded by three oxygen atoms. The carbonate ion is a moderately strong base, so by definition of a Lewis base, it attracts protons in aqueous solutions. It carries a formal charge of -2. Carbonate bonds to metal cations, generally forming insoluble compounds.
Introduction
The term "carbonate" is usually used to refer to one of its salts or carbonate minerals. The more commonly known carbonates are calcium carbonate (\(CaCO_3\)) and sodium carbonate (\(Na_2CO_3\)).
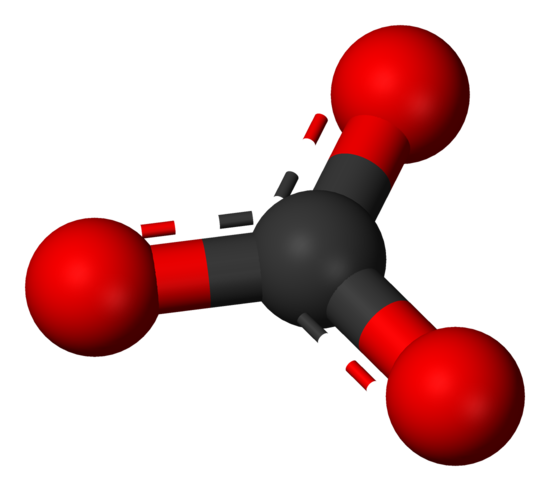
Figure 1. Ball-and-stick model of the carbonate ion: \(CO_3^{2−}\)
Reaction with Group 1 Elements
All of the alkali metals react with carbonate ions and create thermally stable compounds. The exception to that rule is \(Li_2CO_3\). Lithium and magnesium have very similar properties. Their similarities are referred to as a diagonal relationship, possibly due to their comparable size. Therefore, lithium and its compounds do not react the same as other group 1 elements. Some of the examples of alkali metal carbonates are shown below:
- Lithium carbonate, \(Li_2CO_3\): can be used to treat patients who are manic depressive. \[Li^+ + CO_3^{2-} \longrightarrow Li_2CO_3 \nonumber \]
- Sodium carbonate (soda ash), Na2CO3: can be used to manufacture glass.\[Na^+ + CO_3^{2-} \longrightarrow Na_2CO_3 \nonumber \]
Reaction with Group 2 Elements
The group 2 carbonates are the most important minerals of the alkaline earth metals. Their insolubility in water and their solubility in acidic solution makes them ideal reservoirs for petroleum. One of the most significant group 2 carbonates is calcium carbonate, which is the chief constituent of limestone. Limestones are used primarily for building stones including the manufacturing of glasses, Portland cement, and the formation of limestone caves. Here is the reaction of carbonate calcium:
\(Ca^{2+} + CO_3^{2-} \longrightarrow CaCO_3\)
To obtain pure CaCO3 from limestone, three steps must be taken:
- Calcination: decomposing limestone with thermal energy \[CaCO_{3 \; (s)} \longrightarrow CaO_{(s)} + CO_{2 \; (g)} \nonumber \]
- Slaking: adding water to \(CaO_{(s)}\) \[CaO_{(s)} + H_2O_{(l)} \longrightarrow Ca(OH)_{2 \; (s)} \nonumber \]
- Carbonation: Converting \(Ca(OH)_2\) in aqueous form to a precipitated \(CaCo_3\) \[Ca(OH)_{2 \; (s)} + CO_{2 \; (g)} \longrightarrow CaCO_{3 \; (s)} + H_2O_{(l)} \nonumber \]
Practical Applications of Carbonates
Permanent hard water contains HCO3-. By adding Na2CO3 (washing soda), the water is softened and hard water precipitates calcium and magnesium. Ammonium sulfide group filtrate, when treated with CO32-, yields precipitate from the fourth group (Mg, Ca, Sr, Ba). Aqueous carbonate anion is the key reagent, earning the name carbonate group. After the series of precipitations, the solution will contain Na, K, NH4 (common water soluble salts). Bicarbonates are used in the lab to prevent injury or damage from use of strong acids; for instance, by laying out bicarbonate powder in areas of potential acid leakage, accidental spills get neutralized.
Interesting Facts about Carbonates
- Carbonate is a moderately strong base.
- Alkali metals can be mined in the form: Na2CO3, sodium carbonate.
- Except for Li2CO3, alkali metal carbonates are thermally stable.
- Lithium Carbonate was used to treat individuals who are manic depressive.
- Sodium Carbonate (soda ash) is used in the production of glass.
- Calcium Carbonate is limestone.
- Sodium Bicarbonate can be isolated and sold or converted to sodium carbonate by heating.
Outside Links
- For more information on carbonate rocks, click here.
- Hawkes, Stephen J. "Glass Doesn't Flow and Doesn't Crystallize and It Isn't a Liquid." J. Chem. Educ. 2000 77 846.
- Lagier, Claudia; Olivieri, Alejandro. "Calculation of solubilities of carbonates and phosphates in water as influenced by competitive acid-base reactions." J. Chem. Educ. 1990, 67, 934.C
Contributors
- Candice Chan (UCD), Vicky Vo (UCD), Margaret Huang(UCD)

