Silicates
- Page ID
- 39416
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- How to classify silicates?
- What are asbestoses?
A photograph of quartz, one of the many silicates, is shown here. Quartz alone is a very interesting mineral, used in many technologies ranging from jewry to watch, computers, to scientific instrumentation, because quartz is an important piezoelectric material. A clever application of piezoelectric effect is to use its vibration movement to deliver high viscosity ink in the drop-on-demand ink jet system. Piezoelectric materials are also used in Piezo Electric Transducers and Buzzers Techniques have also been developed to apply piezoelectric material for the conversion of static wave energy into rotation motion. Such a Piezoelectric Motor is developed for the wrist watch application.
The silicates are the largest, the most interesting and the most complicated class of minerals than any other minerals. Approximately 30% of all minerals are silicates and some geologists estimate that 90% of the Earth's crust is made up of silicates, SiO44- based material. Thus, oxygen and silicon are the two most abundant elements in the earth's crust.
Silicates is based on the basic chemical unit SiO44-, tetrahedron shaped anionic group. The central silicon ion has a charge of positive four while each oxygen has a charge of negative two (-2) and thus each silicon-oxygen bond is equal to one half (½ ) the total bond energy of oxygen. This condition leaves the oxygens with the option of bonding to another silicon ion and therefore linking one SiO44- tetrahedron to another.
In the extreme case, the tetrahedra are arranged in a regular, orderly fashion forming a three-dimensional network. Quartz is such a structure (see the diagram), and its formula is SiO2. If silica in the molten state is cooled very slowly it crystallizes at the freezing point. But if molten silica is cooled more rapidly, the resulting solid is a disorderly arrangement which is called glass, often also called quartz.
Classify silicates?
For the study of many silicate based minerals, a classification scheme is required. Otherwise, the huge amount of information becomes unmanageable. We use a simple classification scheme based on the number of \(SiO_4^{4-}\) units that are connected together by sharing the oxygen atoms.
Orthosilicates
Orthosilicates are minerals consisting of only single SiO44- units. The cations are some other metals. For example, the following minerals are orthosilicates:
The Be and Zn ions are tetrahedrally bonded to the oxygen of the silicate in these two minerals: phenacite, Be2SiO4 and willemite, Zn2SiO4. In olivine, (Fe, Mg)2SiO4, the cations are either Fe2+ or Mg2+. This formula suggests that this mineral is a mixed salt of iron and magnesium silicates. These cations are octahedrally coordinated to the oxygen atoms of the silicate. Pure salt Fe2SiO4 is called fayalite, and Mg2SiO4 is called forsterite.
Pyrosilicates
When two SiO44- units are linked together, they form the pyrosilicate group, Si2O76-. For example, thortveitite, Sc2Si2O7 is a pyrosilicate.
Ring and chain silicates
When two oxygen of SiO44- units share with other SiO44- units, the silicates form a ring or an infinite chain. The stoichiometry of the silicates becomes (SiO3)n2n-. Benitoite BaTi(SiO3)3 contain three silica rings, but these are relaxed 6-atom rings
SiO2
/ \
O O
| |
O2Si SiO2
\ /
O
The precious stone beryl Be3Al2(SiO3)6 contain six-silica rings.

Single chain silica are called pyroxenes. Some synthetic metasilicates Na2(SiO3) have been shown to contain the simple chain silicates (SiO3)n, in which the Si-O bonds of the type Si-O-Si are 168 nm, with the Si-O-Si angles of 137o. The Si=O bonds are shorter, 1.57 nm. The natural pyroxenes include enstatite, MgSiO3, diopside, CaMg(SiO3)2, and jadeite, NaAl(SiO3)2.
Double chain silicates
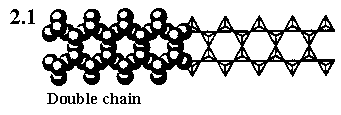 Double chain silicates are called amphiboles, part of the double chain is shown here, same as the double chain shown in Inorganic Chemistry by Swaddle. These chains have a stoichiometry of (Si4O11)n6n. You can easily identify one such unit in the diagram. Tremolite, Ca2Mg5(Si4O11)2(OH)2, is such a mineral.
Double chain silicates are called amphiboles, part of the double chain is shown here, same as the double chain shown in Inorganic Chemistry by Swaddle. These chains have a stoichiometry of (Si4O11)n6n. You can easily identify one such unit in the diagram. Tremolite, Ca2Mg5(Si4O11)2(OH)2, is such a mineral.
The true asbestoses such as crocidolite or blue asbestos consist of double chain silicates. Asbestoses have been identified as carcinogens, and its application has since been limited due to a ban to limit its exposure to the public. Most commercial asbestoses are chrysotile, which contain layers of silicate sheet as we shall below.
Silicates with sheet structures
Sheet slilicates are called phyllosilicates (phyllo means leaflike). These silicates are easy to cleave (as does graphite). Talc is a typical sheet silicate, Mg3(OH)2(Si2O5. Talc is a main ingredient of the soapstone (steatite).
The diagram below shows the arrangement of sheets in brucite, Mg(OH)2, in which the sheets consist of corner sharing octahedrons of Mg(OH)6. In chlorite, there are two types of sheets. Half of the sheets are the same as those of brucite, but half of the brucite-sheets are sandwiched between sheets of silicates. The talc consists of only the sandwiched sheets.
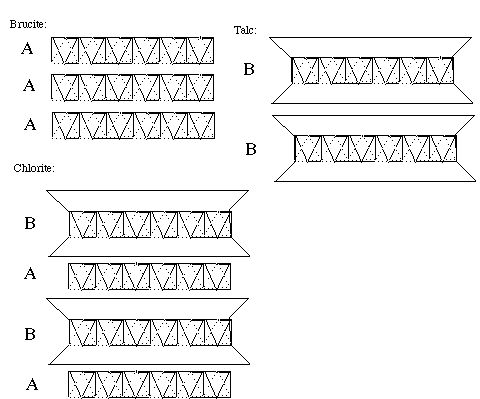
The diagram came from a polysome series which discusses sheet silicates.
Serpentine, Mg3(OH)4Si2O5, has curved sheets.
The comercial asbestos chrysotile is a sheet silicate, but the sheets are rolled up like a tube. These tubes appear as fibers, and they are usually known as asbestos.
Silicates with 3-dimensional framework
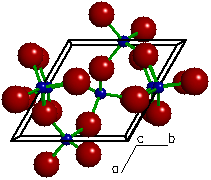 As mentioned earlier, the SiO44- units can share every oxygen with other units to form a three dimensional network, and quartz has such a structure. A portion of such a framework is shown here. In this arrangement, the stoichiometry is reduced to SiO2, which is often called silica. A collection of small pieces of quartz is sand.
As mentioned earlier, the SiO44- units can share every oxygen with other units to form a three dimensional network, and quartz has such a structure. A portion of such a framework is shown here. In this arrangement, the stoichiometry is reduced to SiO2, which is often called silica. A collection of small pieces of quartz is sand.
Please see an animated illustration of the ring structure by Bob Hanson.
Quartz is a group of minerals.
Asbestoses?
Asbestos is the name applied to six naturally occurring minerals that are mined from the earth. The different types of asbestos are:
- Amosite
- Chrysotile
- Tremolite
- ActinoliteUnlink
- Anthophyllite
- Crocidolite
Of these six, three are used more commonly. Chrysotile (white) is the most common, but it is not unusual to encounter. Amosite (brown / off-white), or Crocidolite (blue) as well. Asbestos are noncombustable fibrous material, and they have been used for terminal insulation material, brake linings, construction material, and filters. When mixed with cement, it reinforce the mechanical strength of concrete. It decomposes due to loss of water, and forms forsterite and silica at high temperature.
What is the stoichiometry for an infinite chain of silicates by sharing two oxygen atoms with the neighboring units?
Solution
We can draw such a chain first:
O O O O O O O O O O O O Si Si Si Si Si Si / \ / \ / \ / \ / \ / \ / O O O O O O O
In this diagram, the basic unit is bolded, and the stoichiometry is apparently SiO3.
DISCUSSION
Is there another type of chain that satisfies the condition given in the question? If you work with 3-dimensional models, you probably realize that this is the only way to construct a single chain. Infinite chains and a ring structures have the same stoichiometry.
Questions
- What is the chemical composition of quartz?
Solutions
- SiO4
Skill -
Describe the basic SiO44- unit of silicate and its versatility in connecting to each other or one another. - SiO2
Skill -
Identify the units used to build a structure. - The tetrahedra form a 3-dimensional network like that of a diamond but with linkages of Si-O-Si in places of C-C bonds of diamond.
Skill -
These diagrams age discussed during the lectures. - Minerals consisting of single SiO44- units.
Skill -
Minerals consisting of single Be2+, units are called orthosilicates. These form salts with divalent cations Be2+, Zn2+, Mg2+, Fe2+ etc, but these divalent cations are tightly bonded to the oxygen of the Be2+ units. - Pyrosilicates
- The tetrahedra form a 3-dimensional network like that of diamond but with linkages of Si-O-Si in places of C-C bonds of diamond.
Skill -
These diagrams age discussed during the lectures.
Contributors and Attributions
Chung (Peter) Chieh (Professor Emeritus, Chemistry @ University of Waterloo)

