Aluminosilicates
- Page ID
- 39419
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)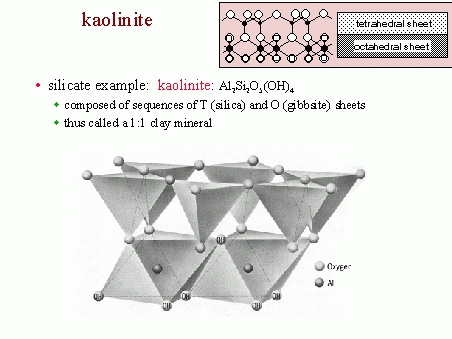
While you are admiring this beautiful picture of faujasite, remember that the oxygen atoms have two unshared electron pairs in addition to the (Al,Si)-O-Si(or Al) bonds. Thus the oxygen atoms are sites to interact with positive site of molecules that passes by these structures. At present over 150 synthetic zeolites & zeotypes and 40 natural zeolites are known. Synthesis of zeolite is a very active field of study.
Aluminosilicates have three major minerals: Andalusite, sillimanite, and kyanite. Zeochem has been developing and manufacturing molecular sieve adsorbents since 1977. Simply put, their adsorbents are used to "screen" out impurities from a variety of applications by attracting and trapping the targeted contaminants. For example, in natural gas processing, molecular sieves are used to remove specific molecules from the gas stream to allow for more efficient downstream processing. Faujasite is a typical zeolite.
Applications of Zeolites?
As you have read above that there are many different kinds of zeolites, each with a definite structure and associate with it are unique properties. In terms of applications, we are assuming zeolites as porous aluminosilicates with large tunnels and cages for a fluid (gas and liquid) to pass through. The applications are based on the interactions between the fluid phase and the atoms or ions of the zeolites. In general terms, zeolites have many applications:
- As selective and strong adsorbers: remove toxic material, selective concentrate a particular chemical, as Molecular Sieve. This link will be a very good to discuss zeolites. Currently, the site is under construction, but it has a very good framework. Even many deorderants are zeolite type.
- As selective ion exchangers: for example used in water softener.
- Superb solid acid catalysts, when the cations are protons H+. As catalysts, their environmental advantages include decreased corrosion, improved handling, decreased environmentally toxic waste and minimal undesirable byproducts.
- As builder: a material that enhance or protecting the cleaning power of a detergent. Sodium aluminosilicate is an ion exchange builder often used in laundry detergent as a builder. A builder inactive the hardness of water by either keeping calcium ions in solution, by precipitation, or by ion exchange.
1 mol CaCO3 2 mol H+ 1 mol z-A 1926 g z-A 100
123 g CaCO3 ----------- ----------- ---------- ---------- ---
100 g CaCO3 1 mol CaCO3 12 mol H+ 1 mol z-A 80
= 494 g zeolite A
That 80 % of protons of the zeolite A is used means that we require a little more zeolite A than stoichiometric quantities.
DISCUSSION
Zeolites are aluminosilicates with open frames strcutures discussed above. Replacement of each Si atom by an Al atom in silicates results in having an extra negative charge on the frame. These charges must be balanced by trapping positive ions: H+, Na K+, Ca2+, Cu2+ or Mg2+. Water molecules are also trapped in the frame work of zeolites.
In this example, we assume that when we soak the zeolite in water containing Ca2+, and Mg2+ ions, these ions are more attrative to the zeolite than the small, singly charged protons. We further assumed that 80 percent of the protons in zeolite are replaced by other ions.
1 mol 0.8*12 mol NaCl 100 58.5 g NaCl
10 kg z-A -------- --------------- --- -----------
2.190 kg 1 mol z-A 20 1 mol NaCl
= 12822 g NaCl = 12.8 kg NaCl
DISCUSSION
How much salt is required if 60% of the sodium ions are effectively used to replace all the divalent ions?
Contributors and Attributions
Chung (Peter) Chieh (Professor Emeritus, Chemistry @ University of Waterloo)

