8.1: Climate Change- Too Much Carbon Dioxide
- Page ID
- 88124
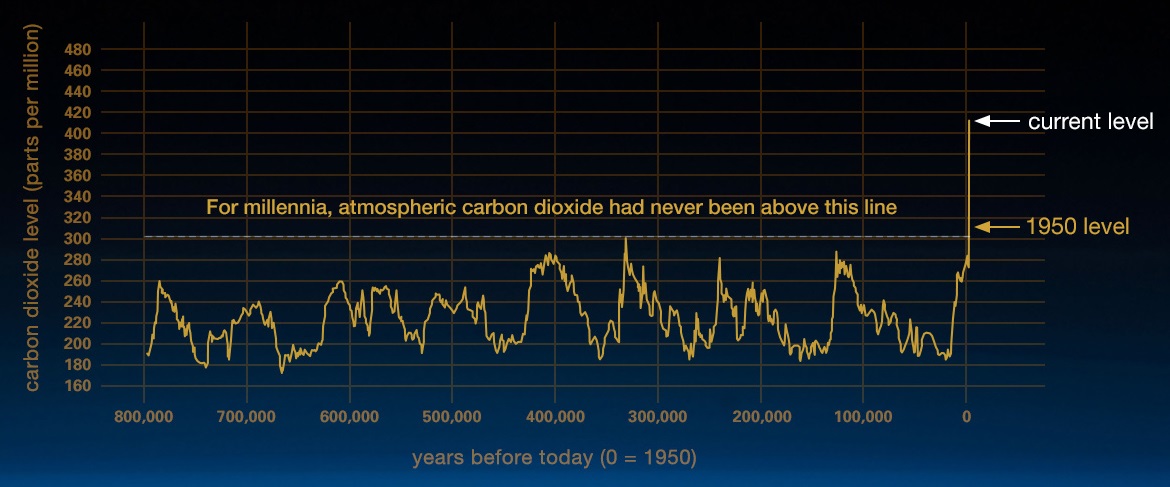
Carbon dioxide (CO2) is an important heat-trapping (greenhouse) gas, which is released through human activities such as deforestation and burning fossil fuels, as well as natural processes such as respiration and volcanic eruptions. Figure \(\PageIndex{1}\) shows CO2 levels during the last three glacial cycles, as reconstructed from ice cores.
Carbon dioxide (\(\ce{CO2}\)) is the primary greenhouse gas emitted through human activities. In 2015, \(\ce{CO2}\) accounted for about 82.2% of all U.S. greenhouse gas emissions from human activities. Carbon dioxide is naturally present in the atmosphere as part of the Earth's carbon cycle (the natural circulation of carbon among the atmosphere, oceans, soil, plants, and animals). Human activities are altering the carbon cycle, both by adding more \(\ce{CO2}\) to the atmosphere and by influencing the ability of natural sinks, like forests, to remove \(\ce{CO2}\) from the atmosphere. While \(\ce{CO2}\) emissions come from a variety of natural sources, human-related emissions are responsible for the increase that has occurred in the atmosphere since the industrial revolution.
The main human activity that emits \(\ce{CO2}\) is the combustion of fossil fuels (coal, natural gas, and oil) for energy and transportation, although certain industrial processes and land-use changes also emit \(\ce{CO2}\). As an example of how \(\ce{CO2}\) can be generated, consider the combustion of octane, a component of gasoline:
\[ \ce{2C8H18 (l) + 21O2 (g) \rightarrow 16CO2 (g) + 18 H2O (g)} \label{eq1} \]
The balanced reaction in Equation \ref{eq1} demonstrates that for every two molecules of octane that are burned, 16 molecules of \(\ce{CO2}\) are generated.
Contributions & Attributions
- Earth Science Communications Team at NASA's Jet Propulsion Laboratory, California Institute of Technology
- EPA

