Glossary
- Page ID
- 279500
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)| Words (or words that have the same definition) | The definition is case sensitive | (Optional) Image to display with the definition [Not displayed in Glossary, only in pop-up on pages] | (Optional) Caption for Image | (Optional) External or Internal Link | (Optional) Source for Definition |
|---|---|---|---|---|---|
| (Eg. "Genetic, Hereditary, DNA ...") | (Eg. "Relating to genes or heredity") |  |
The infamous double helix | https://bio.libretexts.org/ | CC-BY-SA; Delmar Larsen |
| Word(s) | Definition | Image | Caption | Link | Source |
|---|---|---|---|---|---|
| anion, anions | Negatively charged ion | ||||
| aqueous | solution with water as solvent | ||||
| boiling point | Temperature at which vapor pressure of a liquid equals the ambient temperature. (11.6.4) | ||||
| bond dipole moment | A vector representing the separation of charge in a bond that depends on the difference in electronegativity and the distance between the two nuclei being bonded | ||||
| bond dissociation energy, bond dissociation enthalpy | the energy released when one mole of a bond is broken. You must draw Lewis dot structures to identify the bonds (sec 8.9.4). You can use bond dissociation enthalpies to determine enthalpies of reaction. | ||||
| cation, cations | Positively charged ion | ||||
| Clausius-Clapeyron Equation | exponential equation relating vapor pressure of a liquid to the temperature and its enthalpy of vaporization (11.6.3.4) | ||||
| colligative | colligative properties are properties like vapor pressure, boiling and melting points, osmosis, which are affected by adding solutes to a substance (sec 13.5) | ||||
| Compressible | A compressible substance does not resist compression. If you "squeeze" a compressible substance its volume changes. Gases are compressible | ||||
| condense, condensing, condensation | Exothermic phase change where a gas converts to a liquid | ||||
| Coulomb's law | Inverse square law relating the force of interaction between two static charges to the product of the charges and the square of the reciprocal of the distance between them. F=k\(\frac{Q_1Q_2}{r^2}\) | 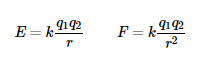 |
|||
| covalent bond | Bond between two nonmetals with equal sharing of electron density. Results when two nonmetals of similar electronegativity (\(\Delta \chi\) <0.5 share electrons in a common orbital | ||||
| critical point | A point on a the liquid-gas equilibrium transition line of a PT phase diagram above which the boundary between the two phases disappear. | ||||
| density | Density in chemistry means the ratio of the mass to the volume of a substance. If a substance is incompressible (solids and liquids), its density can be used to help identify it. Gases on the other hand fill a container, and so their density is not a constant. | ||||
| deposition | Exothermic phase change of a gas directly converting to a solid. | ||||
| dipole, dipole moment | a vector property of a molecule representing the net dipole resulting from the sum of all the bond dipoles in a molecule. Uses the unit of Debye | ||||
| dipole-dipole force, dipole-dipole attraction | intermolecular attraction between two permanent dipoles (section 11.3) | ||||
| dipole-induced dipole | force or attraction between a polar molecule and an adjacent (nonpolar) molecule that it induces a dipole on. (section 11.4.3) | ||||
| distillate | the more volatile coponent that is condensed during distillation (sec 13.1.3) | ||||
| electron affinity | electron affinity is a change in energy when a gaseous atom or ion gains an electron. Electron affinities may be exothermic or endothermic. (sec 7.3.4) | ||||
| electronegativity, electronegative | Concept introduced by Linus Pauling describing the relative power an atom has to attract electrons to itself when bonded to other atoms. For the common elements in the periodic table Fluorine has the highest electronegativity (4) and Cesium and Francium (0.7) have the lowest. High electronegative atoms attract electrons for atoms with lower electronegatively. This is a relative scale and there are no units. The symbol for electronegativity is \(\chi\) | ||||
| endothermic | process where system gains energy from surroundings | ||||
| enthalpy |
The sum of a system’s internal energy U and the product of its pressure P and volume V. (sec 5.4.3) H=U+PV. |
||||
| enthalpy of formation | (ΔHf)o, The standard state enthalpy of formation is the enthalpy change when 1 mole of a substance is formed from it's constituent elements in their standard state. The standard state is 1 bar and 298K. (sec. 7.7.3) these values can be obtained in a Standard Table of Thermodynamic Properties | ||||
| exothermic | process where system releases heat to the surroundings | ||||
| formal charge | The charge that would result on an atom due to the number of valence electrons it adds a molecule minus the number actually around it (add electrons donated minus the number of bonds and lone pair electrons). (section 8.4) | ||||
| freeze, freezing | exothermic phase change from liquid to solid. | ||||
| fusion | the same as melting (when referring to physical changes) | ||||
| hydrogen bond, hydrogen bonds | intermolecular force that exists when hydrogen is bonded to a strongly electronegative atom like F, O or N. The electronegative atom must have a lone pair of electrons, which are attracted to the hydrogen of a different bonding pair((\(\delta\)+). (11.5) | ||||
| hydrophilic | water loving- polar or charged part of a molecule that is soluble in water | ||||
| hydrophobic | water fearing - nonpolar part of a molecule that does | ||||
| ion-dipole | Intermolecular force of attraction between an ion and a polar molecule (section 11.2) | ||||
| ionization energy | Ionization energy is the energy required to move an electron from a lone atom or ion in the gas phase. Ionization energies aer always endothermic (sec 7.3.3) | ||||
| incompressible | Something that resists compression. If you "squeeze" and incompressible substance its intermolecular forces prevent its volume from contracting, and so it has a relatively constant density. Solids and liquids are typically considered to be incompressible. | ||||
| induced dipole | dipole induced on a non polar molecule by an external field, which could be from an adjacent dipole. | ||||
| instantaneous dipole-induced dipole | short range force of attraction between two non polar molecules where an instantaneous dipole in one molecule induces a dipole in another. These are often called dispersive or London dispersion forces. (section 11.4.4) | ||||
| ionic bond | Bond resulting from net attractive force of positive cations to negative anions. | ||||
| isobar, isobaric | system at constant pressure, | ||||
| isotherm, isothermic | system at constant temperature | ||||
| kinetic molecular theory | The kinetic molecular theory describes an ideal gas (10.5) | ||||
| melting | endothermic phase change from a solid to a liquid | ||||
| nonvolatile | substance that does not have a vapor pressure above its surface. | ||||
| normal boiling point | boiling point when vapor pressure above a liquid equals 1 atm | ||||
| oscillation | Movement back and forth at constant speed. | ||||
| polar bond, polar covalent bond | bond between two atoms of different electronegativity. | ||||
| Polarizability | measure of the ability of an external electric field (charge) to distort a molecules charge distribution (electron cloud) (section 11.4.2) | ||||
| polar molecule | A molecule with a net overall dipole | ||||
| solute | In a liquid phase solution the solute is the substance that is dissolved into the solvent by intermolecular forces. There can be more than one solute in a solution | ||||
| solution | homogenous mixture | ||||
| solvent | In a liquid phase solution the solute is the substance present in greatest proportions. Aqueous solutions have water as the solute, even if it is not in the greatest proportions. | ||||
| sublimation | Endothermic phase change of a solid to a gas. Dry ice (solid CO2) sublimes | ||||
| supercritical fluid | At high temperature and high pressure the boundary between a liquid and gas disappears and you have a supercritical fluid (sec sec 11.6.5 and sec 11.5.2) | ||||
| temperature | Temperature is an intensive property of matter related to its "hotness" or "coldness" | ||||
| triple point | A point on the PT phase diagram where 3 phases can coexist in equilibrium. | ||||
| Van der Waals force, Van der Waals forces, Van der Waals | Weak, short range attractive forces between molecules, including dipole-dipole, dipole induced dipole and London dispersion forces (section 11.4.5) | ||||
| vapor pressure | Two meanings. We will normally say the vapor pressure is the equilibrium vapor pressure when the rate a volatile substance evaporates off a surface equals the rate the gas phase particles condense onto the surface. It also just means the pressure due to the particles in the gas phase. (11.6.3) | ||||
| viscosity | measure of liquids resistance to flow | ||||
| volatile | substance with a vapor pressure above its surface ( substance that evaporates or sublimes) |

