13.1: Prelude
- Page ID
- 408736
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Introduction:
Solutions are homogenous mixtures of two or more compounds. They may be solid phase (alloys), liquid phase or gas phase. In the case of liquid phase solutions, the solvent is typically the compound in greater quantity, and the solute in lesser, where the solute is dissolved into the solvent. In aqueous solutions water is the solvent, even if it is in lesser quantities, and in this class we will be dealing mostly with aqueous solutions. The solute can be of a different phase than the solvent
- Gas Phase - like the air, the idea of a solute dissolved in a solvent does not fit this. Gaseous systems are typically described by the partial pressure and mole fraction, review Raoult's Law from your gas phase chapter.
- Liquid Phase - typically described by solutes dissolved in solvent.
Solute can be- Liquid (alcohol in wine)
- solid (salt or sugar in water)
- gas (oxygen in water or blood)
- Solid Phase (may be amorphous or crystalline
If Crystalline- Substitutional (solute takes place of atoms in crystal lattice)
- Interstitial (solute is in voids of crystal)
Solubility
Solubility is a quantitative measurement of how much of a solute can be dissolved in a solvent. We will go over the units of concentration in the next section. The term solubility often refers to the concentration of a saturated solution
Saturated Solution
A saturated solution has the maximum amount of solute that can exist in equilibrium with undissolved solute. If you add more solute to a saturated solution it does not dissolve, typically falling to the bottom as a precipitate and the concentration of the solution does not change.
Unsaturated Solution
An unsaturated solution can dissolve more solute, and so as you add solute, the concentration of the dissolved particles increases.
Supersaturated Solution
A supersaturated solution is a metastable state which contains more dissolved solute than can exist in equilibrium with undissolved solute. If a small seed crystal is added the dissolved solute in excess of a saturated solution crashes out as is shown in the following video. A metastable state is a state that can be disrupted by a minor disturbance or perturbation. For example, scratching the sides of the glass with a piece of metal could cause the crystals to form.
Video \(\PageIndex{1}\): Supersaturated solution of Sodium Acetate uploaded by dchummer Chemistry (https://youtu.be/FcxZ9DyOaUk)
Liquid/Liquid Solutions
Miscible Solutions: Soluble in all proportions
Immiscible Solutions: Insoluble, do not mix and form separate layers
Is there an easy way to determine if two liquids are miscible or immiscible?
Like Dissolves in Like:
- Polar liquids dissolve in polar liquids
- Nonpolar liquids dissolve in nonpolar liquids

Figure \(\PageIndex{1}\): Separatory funnels showing immisicible solutions, the bottom layer are aqueous solutions with food coloring added, and the top layer is an organic solvent. The dye molecules are polar and so dissolve in the polar aqueous layer, coloring that layer while making the non-polar layer clear.
The two solutions in the above separatory funnel are immiscible, and so they form different phases. In this case we are looking at a nonpolar organic solvent with a low density. If the organic solvent was a dense solution like carbon tetrachloride, the clear layer would be at the bottom.
How can you separate two miscible liquids?
Distillation
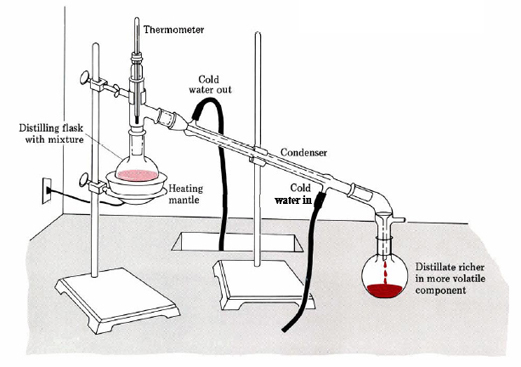
Figure \(\PageIndex{2}\): Distillation apparatus for separating two miscible liquids that have different boiling points.
Two substances with different boiling points are placed in the distilling flask (above figure). As heat is applied, the vapor pressure of the two substances increases and their gas phase molecules rise up and enter the condenser, which cools them, causing condensation and they drip into the collection flask, forming the distillate. At some point the boiling point of the more volatile substance is reached, which then boils and the system stays at its boiling point until it is all boiled. At this point the two compounds have been separated with the more volatile compound being the distillate and collected in the collection flask, while the less volatile compound stays in the distillation flask. It should be noted that the distillate is not pure as some of the less volatile compound vaporized, and this can be further distilled, producing increasingly pure distillate on the second or third distillation.
Video\(\PageIndex{1}\): 1:45 min YouTube on Fractional Distillation developed by the Royal Society of Chemistry.
Exercise \(\PageIndex{1}\)
Would octane (C8H18) and Pentane (C5H12) be miscible or immiscible?
- Answer
-
They are both nonpolar, and would be miscible.
Exercise \(\PageIndex{2}\)
If you placed octane and pentane in a still, which would be the distillate?
- Answer
-
Octane has the higher enthalpy of vaporization and lower vapor pressure, and so Pentane would have the higher vapor pressure and boil first. There are two easy to understand reasons why it has a higher vapor pressure (and lower enthalpy of vaporization). First, it is lighter in mass, and so it is easier to vaporize. Second, pentane has weaker intermolecular forces, being smaller in volume, it's valence electrons are held in tighter, making it less polarizable, and so it would have weaker instantaneous-induced dipole intermolecular forces (London Dipsersive Forces) to hold it together..
How do you separate two immiscible fluids?
One way to do this is through a separatory funnel (figure 13.0.1), where the more dense layer is the bottom layer. Note, "like dissolves in like" does not just apply to liquids dissolved in liquids, but also to various solutes. This explains why ionic compounds can be dissolved in water, and so extraction techniques often involve isolating a product from other compounds by extracting it into a solution for which it is preferentially soluble.
Solvent Phase Extraction: Process by which a solute phase product can be extracted from a reaction mixture through its affinity for one of two immiscible liquids.
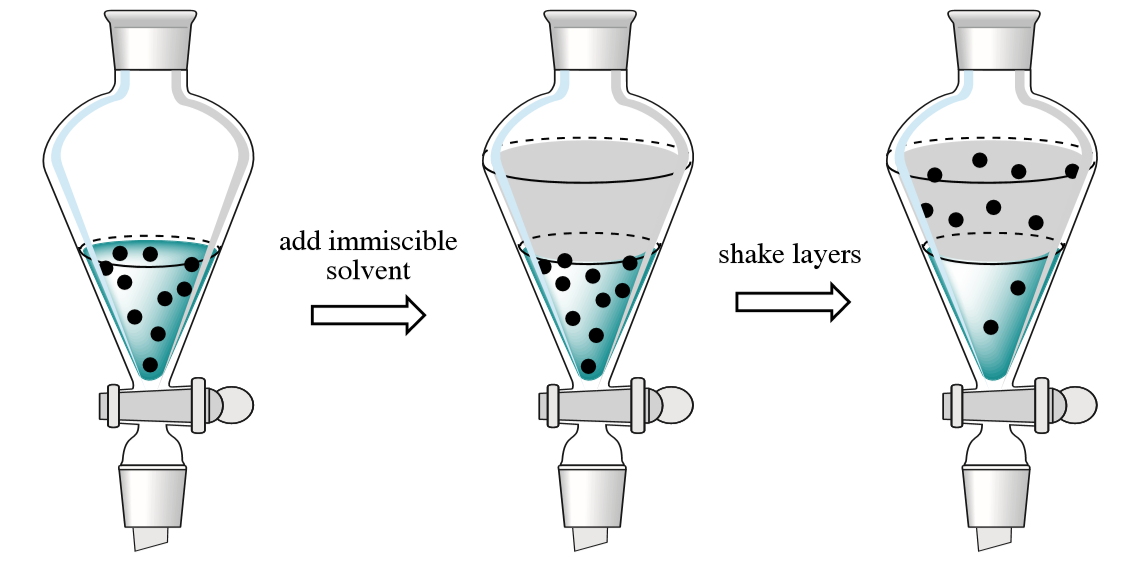
Figure \(\PageIndex{2}\): Consider on the left for the black dots to be the nonpolar product of an aqueous phase reaction, like that between iodide and ferric iron to produce iodine and ferrous iron (eq. 13.0.1). If an immiscilbe nonpolar solvent was added, the nonpolar iodine would have a higher solubility in it than in the polar water phase, and thus would transfer across the boundary and leave the aqueous phase for the organic phase. This could allow you to extract the iodine.
Consider the equation
\[2Fe^{+3} + 2I^{-} \rightarrow 2Fe^{+2} + I_{2}\]
The reactants (Fe+3 and I-) are water soluble through ion-dipole interactions, and one of the products (Fe+2) is also water soluble, but the other product, molecular iodine (I2) is nonpolar and so is not very soluble in water, but would be soluble in a nonpolar solvent like carbon tetrachloride. So a reaction between water soluble reactants could produce a nonwater soluble product, that in turn could be extracted by dissolving it in a nonpolar solvent and then extracting through a separatory funnel.
Video\(\PageIndex{2}\): 3:42 min YouTube on solvent separation with a separatory funnel developed by the Royal Society of Chemistry.
Colligative Properties
- properties of a solvent like the boiling and melting point which for an ideal solution are influenced by the quantity of solute particles, and not the chemical identity of the solute particles. These are covered in section 13.5
Colloids
- homogenous mixtures like solutions, but metastable, that is, they will eventually break into a heterogenous mixture. A foam is a homogeous colloid made of a gas in a liquid, and will eventually fall apart, while oxygen dissolved in water is a pure solution, and will not break. These are covered in section 13.6.
In Class Activities
ADAPT \(\PageIndex{1}\)
Contributors and Attributions
Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The breadth, depth and veracity of this work is the responsibility of Robert E. Belford, rebelford@ualr.edu. You should contact him if you have any concerns. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to:
- Lisa Nichols (images repurposed from LibreText site)
- Anonymous

