Agenda Topics
- Page ID
- 174170
Welcome to the Fall 2019 General Chemistry 1 LibreText Agenda Topics.
This page will be updated as the semester proceeds.
Periodic Table
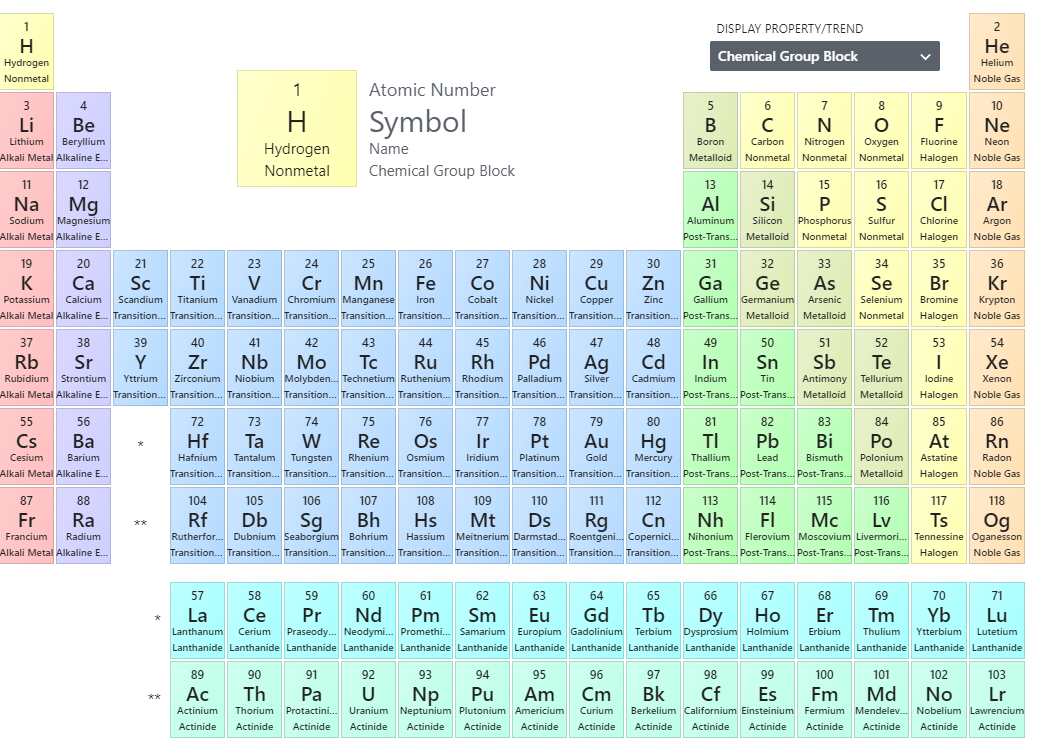
Image of PubChem Periodic Table of the Elements: https://pubchem.ncbi.nlm.nih.gov/periodic-table/
Overview of Daily Lectures
Lecture 1: 8/20/2019
Agenda for First Period (Obviously not flipped)
- Introduction to Class
- Cover Chapter 1A
- hypothes.is
- Hypothes.is Group: 2019FC1402 (case sensitive)
- Memorize NON SHADED Elements on this Periodic Table (Quiz on 3rd period of class)
Lecture 2: 8/22/2019
This chapter reviews material dealing with mathematical calculations and the representation of measured values. It is important to watch the videos and skim through the reading before class.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
These worksheets are from Chem 1300 (PrepChem) and will be used to review this material. Worksheets 1 & 2 will not be covered in lecture. Select problems from worksheets 3-5 will |
Assumed Knowledge
- In principle this chapter is a review, but we will show some shortcuts that will make things easier.
Lecture 3: 8/27/2019
Continuation of Unit Conversions and Dimensional Analysis
ad.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
These worksheets are from Chem 1300 (PrepChem) and will be used to review this material. Select problems from worksheet 5 will be covered.
|
Assumed Knowledge
- In Principle this chapter is a review, although the Plus 40/minus 40 technique is probably new, and worth learning.
Lecture 4: 8/29/2019
Atoms, isotopes and isotopic abundances.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
|
Assumed Knowledge
- fractions
Lecture 5: 9/03/2019
In this section we will cover basic covalent and ionic bonds, and the determination of ionic formulas. You need to start memorizing polyatomic ions, and some tips are in the next section, so we will be reading from that too.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
Assumed Knowledge
Lecture 6: 9/05/2019
In this section we will cover basic nomenclature, the naming of ionic and covalent compounds, including simple moleculde, salts, acids, acid salts and hydrated salts.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
Assumed Knowledge
- Formula of Ionic Compounds, names of elements
Lecture 7: 9/10/2019
The lecture will go over mass and molar composition of substances.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
|
Assumed Knowledge
- understand fractions and percents, dimensinal analysis
Lecture 8: 9/12/2019
Types of chemical Equations and begin balancing equations
ad.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
|
Assumed Knowledge
- chemical nomenclature and formula
Lecture 9: 9/17/2019
Balancing Chemical Equations
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets | |
|---|---|---|---|
|
3.4 Aqueous Reactions 3.4.1-3.4.2.2 3.5 Acid/Base Chemistry
|
|
Assumed Knowledge
- Types of reactions, nomenclature and balancing equations
Lecture 10: 9/19/2019
Aqueous Reactions and Acid Base reactions
ad.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
3.6 Redox Chemistry
|
3.6 Redox
|
Assumed Knowledge
- Predict double displacement reaction with solubility rules.
Lecture 11: 9/24/2019
Complete Chapter 3 with Redox and review
ad.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
Assumed Knowledge
- TBA
Lecture 12: 9/26/2019
Exam I
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
4.1 Stoichiometry
|
4.1 Stoichiometry 4.2 Limiting & Excess Reagents 4.3 Percent Yield |
Assumed Knowledge
- Everything we covered in class.
Lecture 13: 10/01/2019
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
Assumed Knowledge
- Balancing equations and basic stoichiometry
Lecture 14: 10/03/2019
Energy is the capacity to supply heat or do work. Two ways to transfer energy: Heat (Originate from difference in temperatures (i.e., average kinetic energy of molecules)) and Work (moving an object against a resistance (e.g., expansion work of a gas)). Mechanical work: \(w=Fd\), Expansion (PV) Work: w=PΔV (under constant pressure).
ad.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
Assumed Knowledge
- Algebra
Lecture 15: 10/08/2019
Energy is the capacity to supply heat or do work. Two ways to transfer energy: Heat (Originate from difference in temperatures (i.e., average kinetic energy of molecules)) and Work (moving an object against a resistance (e.g., expansion work of a gas)). Mechanical work: \(w=Fd\), Expansion (PV) Work: w=PΔV (under constant pressure). Energy is fluid (but not a real fluid) that can be converted where Kinetic energy can be interconverted with Potential Energy.
ad.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
|
Assumed Knowledge
- TBA
Lecture 16: 10/10/2019
Now that enthalpy and state functions have been introduced, we will use them to calculate the enthalpies of reactions. We will also apply the First Law to calorimetry problems.
ad.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
|
Assumed Knowledge
- TBA
Lecture 17: 10/15/2019
We will finish the chapter on thermo and start the chapter of the electronic structure of the atom.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
|
Assumed Knowledge
- TBA
Lecture 18: 10/17/2019
We will cover quantum theory and the types of orbitals used to describe electron distributions
ad.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
|
Assumed Knowledge
- TBA
Lecture 19: 10/22/2019
We need to complete chapter 6 and start chapter 7. These are very related to each other, and in some textbooks are parts of the same chapters
ad.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
|
Assumed Knowledge
- TBA
Lecture 20: 10/24/2019
Finish Chapter 7
ad.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
|
Assumed Knowledge
- TBA
Lecture 21: 10/29/2019
Energy is the capacity to supply heat or do work. Two ways to transfer energy: Heat (Originate from difference in temperatures (i.e., average kinetic energy of molecules)) and Work (moving an object against a resistance (e.g., expansion work of a gas)). Mechanical work: \(w=Fd\), Expansion (PV) Work: w=PΔV (under constant pressure). Energy is fluid (but not a real fluid) that can be converted where Kinetic energy can be interconverted with Potential Energy.
ad.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
|
Assumed Knowledge
- TBA
Lecture 22: 10/31/2019
Energy is the capacity to supply heat or do work. Two ways to transfer energy: Heat (Originate from difference in temperatures (i.e., average kinetic energy of molecules)) and Work (moving an object against a resistance (e.g., expansion work of a gas)). Mechanical work: \(w=Fd\), Expansion (PV) Work: w=PΔV (under constant pressure). Energy is fluid (but not a real fluid) that can be converted where Kinetic energy can be interconverted with Potential Energy.
ad.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
|
Assumed Knowledge
- TBA
Lecture 23: 11/05/2019
Energy is the capacity to supply heat or do work. Two ways to transfer energy: Heat (Originate from difference in temperatures (i.e., average kinetic energy of molecules)) and Work (moving an object against a resistance (e.g., expansion work of a gas)). Mechanical work: \(w=Fd\), Expansion (PV) Work: w=PΔV (under constant pressure). Energy is fluid (but not a real fluid) that can be converted where Kinetic energy can be interconverted with Potential Energy.
ad.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
|
Assumed Knowledge
- TBA
Lecture 24: 11/07/2019
Energy is the capacity to supply heat or do work. Two ways to transfer energy: Heat (Originate from difference in temperatures (i.e., average kinetic energy of molecules)) and Work (moving an object against a resistance (e.g., expansion work of a gas)). Mechanical work: \(w=Fd\), Expansion (PV) Work: w=PΔV (under constant pressure). Energy is fluid (but not a real fluid) that can be converted where Kinetic energy can be interconverted with Potential Energy.
ad.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
|
Assumed Knowledge
- TBA
Lecture 25: 11/12/2019
Energy is the capacity to supply heat or do work. Two ways to transfer energy: Heat (Originate from difference in temperatures (i.e., average kinetic energy of molecules)) and Work (moving an object against a resistance (e.g., expansion work of a gas)). Mechanical work: \(w=Fd\), Expansion (PV) Work: w=PΔV (under constant pressure). Energy is fluid (but not a real fluid) that can be converted where Kinetic energy can be interconverted with Potential Energy.
ad.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
|
Assumed Knowledge
- TBA
Lecture 26: 11/14/2019
Energy is the capacity to supply heat or do work. Two ways to transfer energy: Heat (Originate from difference in temperatures (i.e., average kinetic energy of molecules)) and Work (moving an object against a resistance (e.g., expansion work of a gas)). Mechanical work: \(w=Fd\), Expansion (PV) Work: w=PΔV (under constant pressure). Energy is fluid (but not a real fluid) that can be converted where Kinetic energy can be interconverted with Potential Energy.
ad.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
|
Assumed Knowledge
- TBA
Lecture 27: 11/19/2019
Energy is the capacity to supply heat or do work. Two ways to transfer energy: Heat (Originate from difference in temperatures (i.e., average kinetic energy of molecules)) and Work (moving an object against a resistance (e.g., expansion work of a gas)). Mechanical work: \(w=Fd\), Expansion (PV) Work: w=PΔV (under constant pressure). Energy is fluid (but not a real fluid) that can be converted where Kinetic energy can be interconverted with Potential Energy.
ad.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
|
Assumed Knowledge
- TBA
Lecture 28: 11/21/2019
Energy is the capacity to supply heat or do work. Two ways to transfer energy: Heat (Originate from difference in temperatures (i.e., average kinetic energy of molecules)) and Work (moving an object against a resistance (e.g., expansion work of a gas)). Mechanical work: \(w=Fd\), Expansion (PV) Work: w=PΔV (under constant pressure). Energy is fluid (but not a real fluid) that can be converted where Kinetic energy can be interconverted with Potential Energy.
ad.
| Before Class Readings | Lecture Topics | Assignments/In class Worksheets |
|---|---|---|
|
|
|
Assumed Knowledge
- TBA

