1A.3: Classifying Matter
- Page ID
- 50453
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Identify the four states of matter
- Compare and contrast the characteristics of solids, liquids, and gases
- Classify substances as pure substances or mixtures
- Classify substances as homogenous and heterogeneous, or as elements or compounds
What are the Three Attributes of Matter?
- Matter has Mass
- Matter has Volume
- Matter has Energy
What are the states of matter?
Matter has mass, occupies space and exists in different states that are determined by its energy. There are four fundamental states of matter that are observable in everyday life: solid, liquid, gas, and plasma. However, other states are known to exist in extreme situations (e.g., low or high energy states, as discussed in this Wikipedia article). In this class we will study the first three, but you need to be aware of all four of the fundamental states.
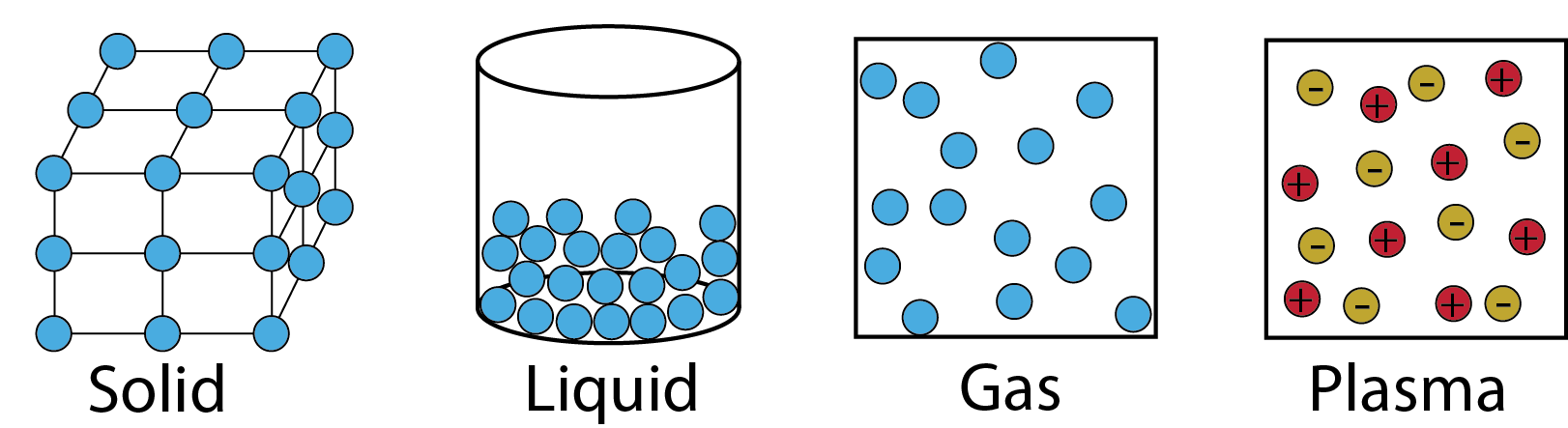
Solid
Incompressible Rigid State: Constituent atoms or molecules do not move with respect to each other, but can vibrate in their positions.
- Resists change in density (density is typically constant).
- Maintains shape (structural rigidity).
Liquid
Incompressible Fluid State
- Resists change in density (denisty is typically constant).
- Not rigid, will flow and take shape of container.
Gas
Compressible Fluid State
- Variable density.
- Fills entire container.
Is a gas a Fluid?
Yes, a gas is a fluid.
Plasma
- High energy state where atoms can lose an electron and both species coexist.
- Most of the matter of the universe is in the plasma state.
The following video gives a good description of the plasma state.
What Factors Determine the Stable Phase of Matter?
This is a rather complicated question that we will deal with as the course proceeds. Essentially, it is an interplay between the cohesive forces of attraction which hold the particles together and the kinetic energy of motion. We will learn later that the Kinetic Molecular Theory states that the Kinetic Energy is proportional to the temperature, so as you increase the temperature you increase the kinetic energy of matter. At "low temperatures" the cohesive forces dominate and the solid phase is stable. As you add heat the temperature rises until you reach the melting point. Continued heat causes melting, and once you have melted the entire substance the liquid phase increases its temperature until it reaches it's boiling point. At the boiling point continued head causes vaporization and the temperature does not increase until it is all a gas. So the stable phase depends on the temperature, and the forces holding a substance together.
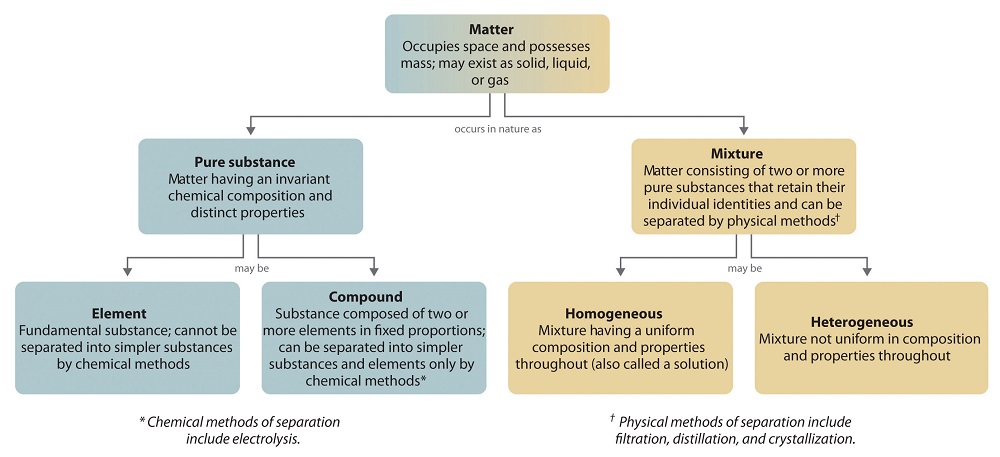
Mixtures and Pure Substances
Homogenous Matter - of same form. From Greek homos (same) and genos (race/kind)
Heterogeneous Matter - of different form. From Greek heteros (other) and genos (race/kind)
What is the Difference Between a Pure Substance and a Mixture?
A pure something has one entity, it could be an element or a compound. Pure water, pure table salt (sodium chloride) or pure gold are all pure substances. But a combination of water and salt or water and gold are mixtures. Note, if all the salt dissolves it is a homogeneous mixture, but a mixture of water and gold (which is insoluble) is heterogeneous.
Which is a Solution, a Homogenous or Heterogeneous mixture?
A solution is a homogenous mixture. Examples are milk, wine and ocean water. But a mixture of sand and water is not a solution because the sand does not dissolve, and so is a heterogenous mixture.
Can a pure Substance be Heterogenous?
Yes, for example ice water is heterogeneous with the two phases having distinctly different properties like density, although based on the definition many chemists use, it is not a mixture, as both ice and water are the same substance, the molecule H2O. But even if it is treated as a pure substance, it is still heterogenous. Note this two phase system is an unstable mixture at any temperature above or below the melting point, as above the melting point the ice will melt, and below the melting point the water will freeze. At the melting point the system is stable if it is energetically isolated from the environment, which if you think about it, is a very hard thing to do. If heat enters enters the system at the melting point, some of the ice will melt, and if it leaves, some of the liquid water will freeze.
Note, the above discussion leads one to ask, is a mixture of ice and water a mixture? I personally say yes, as they have distinct properties, but I know of other chemists who say no, as they are both the same substance, water. This shows that even simple schema for classifying matter as a pure substance or mixture can lead to confusion, and you might want to ask your instructor what they think, and what definition of a pure substance they use.
Schema for Classification of Matter
The following flow chart breaks matter down into pure substances and mixtures, and note that it does not take into the account that a pure substance can exist as a mixture of phases with distinct properties.
How can you Separate a Heterogeneous Mixture?
- Filtration- allows an insoluble solid to be separated from a liquid phase.

Figure \(\PageIndex{3}\): Illustration of suction filtration lab set up for organic chemistry, that allows separation of precipitate (solid) from a solvent/solution that it was formed in.
- Magnets - if one substance is magnetic and the other not, a magnet will separate them.
Video \(\PageIndex{2}\): YouTube showing how to separate iron from cereal (https://youtu.be/oQ5lzpAw2qE) uploaded by BBC Science Focus Magazine
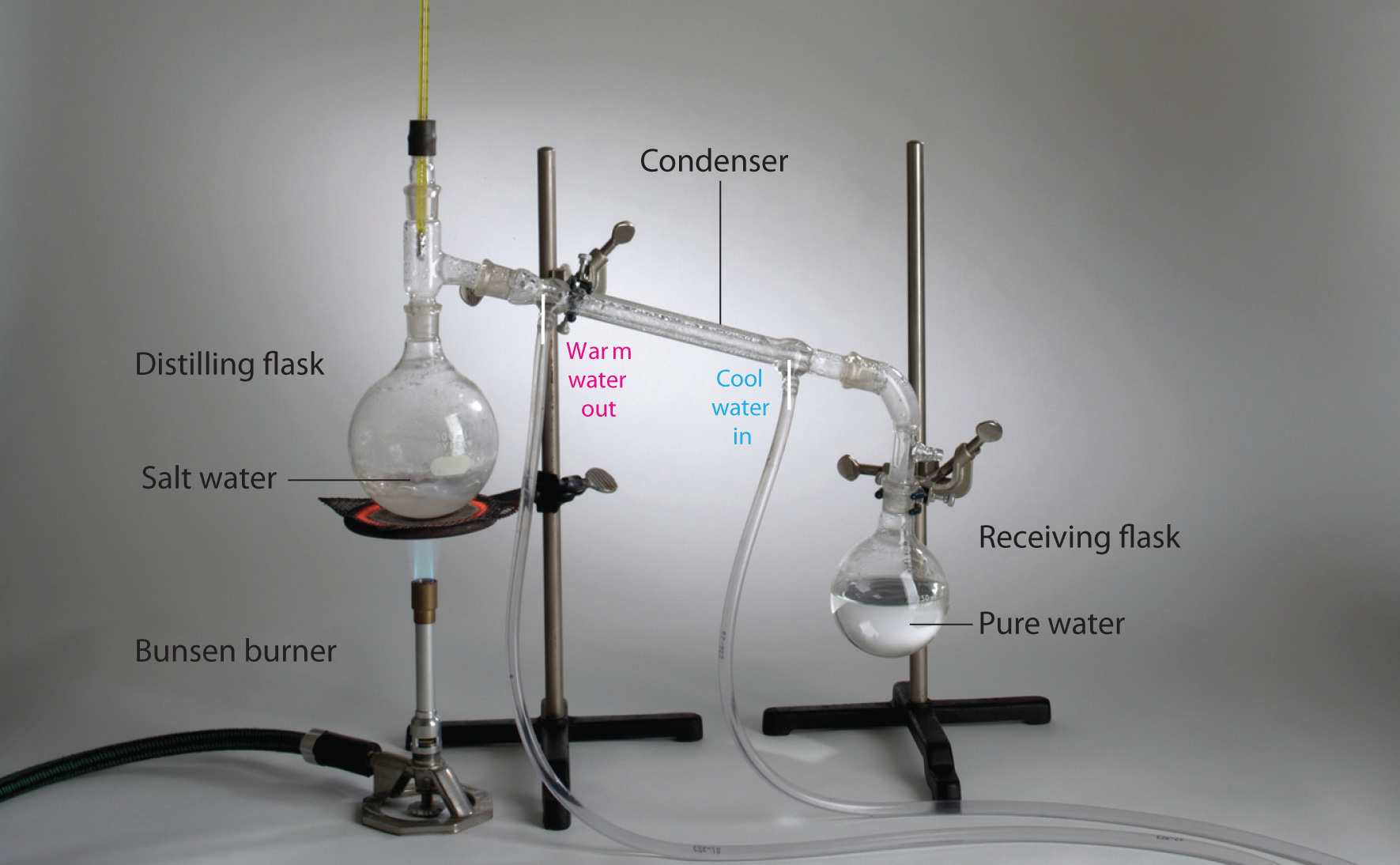
How can you Separate a Homogenous Mixture?
Distillation
if one substance has a different boiling point than the other, it will boil off first. A still is as a device that can be used to separate two substances. Note, if both substances are volatile, like in an alcohol still where ethanol and water can be separated, the ethanol which has the lower boiling point boils off first, but the distallate (material collected in the receiving flask) will have a small amount of water, because water does have a vapor pressure below its boiling point (that is why water in an open container evaporates). If you want really pure ethanol you would double or triple distil.
Chromatography
Chromatography was developed around the turn of the last century and originally used to identify the pigments in plants. In the following video the girl breaks up plant leaves and soaks them in alcohol (a heterogeneous mixture). She then strains them and has a green homogenous mixture (solution). She then places a strip of filter paper into the solution with most of the strip above the surface. As the solvent moves up the filter paper it drags the pigments with them, but due to their different chemical properties they travel at different rates and breakup into bands.
In the next sections we will look at elements and compounds.
Test Yourself
Query \(\PageIndex{1}\)
Contributors and Attributions
Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The breadth, depth and veracity of this work is the responsibility of Robert E. Belford, rebelford@ualr.edu. You should contact him if you have any concerns. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to:
- Material modified by Joshua Halpern (Howard University), Scott Sinex, and Scott Johnson (PGCC)
- Lisa Nichols (images, Butte Community College)
- Elena Lisitsyna (H5P interactive modules)


