1B: Review of the Tools of Quantitative Chemistry
- Page ID
- 158499
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
SI Units
Exercise \(\PageIndex{1}\)
The SI base unit of length is the ____.
- newton
- liter
- meter
- candela
- Ångstrom unit
- Answer
-
- meter (see 1B.3.1: SI Base Units)
Exercise \(\PageIndex{2}\)
The SI base unit of temperature is the ____.
- Kelvin
- calorie
- Fahrenheit
- Ångstrom
- kilocalorie
- Answer
-
- Kelvin (see 1B.3.1: SI Base Units)
Exercise \(\PageIndex{3}\)
Which of the following is not a base SI unit?
- meter
- kelvin
- calorie
- second
- mole
- Answer
-
- calorie (see 1B.3.1: SI Base Units)
Exercise \(\PageIndex{4}\)
The absolute zero point on the Kelvin scale is equal to _____.
- –273.15 °C
- 0 °C
- 14.5 °C
- 25 °C
- –373.15 ⁰C
- Answer
-
- –273.15 °C (see 1B.5.2 Temperature Conversions)
SI Prefixes
Exercise \(\PageIndex{5}\)
Give the name of the prefix and the quantity indicated by the following symbols that are used with SI base units.
- c
- d
- G
- k
- Answer a
-
centi-, × 10−2
- Answer b
-
deci-, × 10−1
- Answer c
-
Giga-, × 109
- Answer d
-
kilo-, × 103
Exercise \(\PageIndex{6}\)
Give the name of the prefix and the quantity indicated by the following symbols that are used with SI base units.
- m
- n
- p
- T
- Answer a
-
milli-, × 10−3
- Answer b
-
nano-, × 10−9
- Answer c
-
pico-, × 10−12
- Answer d
-
tera-, × 1012
Experimental Error
Exercise \(\PageIndex{7}\)
The following archery targets show marks that represent the results of four sets of measurements. Which target shows
- a precise but inaccurate set of measurements?
- an accurate but imprecise set of measurements?
- a set of measurements that is both precise and accurate?
- a set of measurements that is neither precise nor accurate?
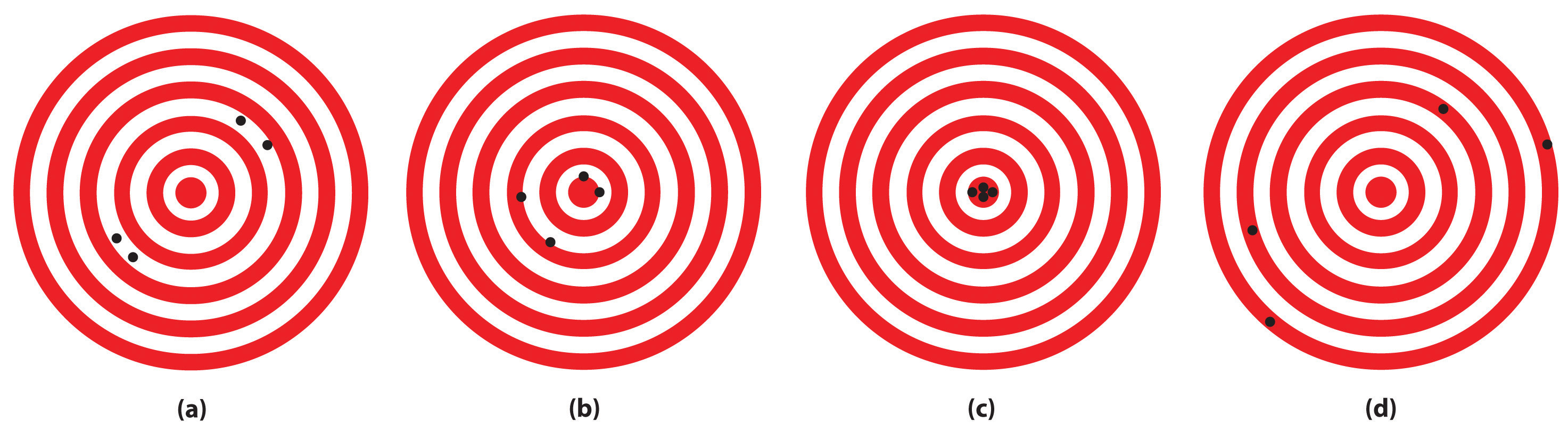
- Answer
-
- B
- A
- C
- D (see 1B.2:1.1 Accuracy, 1B.2:1.2 Precision)
Exercise \(\PageIndex{8}\)
Classify the following sets of measurements as accurate, precise, both, or neither.
- Checking for consistency in the weight of chocolate chip cookies: 17.27 g, 13.05 g, 19.46 g, 16.92 g
- Testing the volume of a batch of 25-mL pipettes: 27.02 mL, 26.99 mL, 26.97 mL, 27.01 mL
- Determining the purity of gold: 99.9999%, 99.9998%, 99.9998%, 99.9999%
- Answer
-
a. neither
b.precise
c.both (see 1B.2:1.1 Accuracy, 1B.2:1.2 Precision)
Significant Figures
Exercise \(\PageIndex{9}\)
Give the number of significant figures in each measured value.
- 5.87
- 0.031
- 52.90
- 0.2001
- 500
- Answer a
- Answer b
- Answer c
- Answer d
- Answer e
-
1 (see 1.B2:2.1 Significant Figure Rules), note, some texts will say it is not determined. That is, you do not know. We will use the convention that trailing zeros are not significant if there is not a decimal, but if your instructor uses a different convention, play the game and use the rules your class uses.
Exercise \(\PageIndex{10}\)
Give the number of significant figures in each measured value.
- 0.0053
- 2300
- 32.00
- 34.483
- 3450000
- Answer a
- Answer b
- Answer c
- Answer d
- Answer e
SI Prefix Conversion
Exercise \(\PageIndex{1}\)
Convert the following:
- 32.4 ng to _____ Mg
- 56.4 T bytes to _____ M bytes
- Answer a
-
3.24 x 10-14 Mg
\[ 32.4ng \frac{10^{-6}Mg}{10^9ng} = 3.24*10^{-14}Mg \\ \text{or the long way} \\ (32.4ng)(\frac{10^{-9}g}{1 ng})(\frac{1Mg}{10^{6}g})=32.4*10^{(-9-6)}Mg=32.4*10^{-15}Mg= 3.24*10^{-14}Mg \]
Note: You should be able to do this the first way without writing everything out (you add exponents on the numerator and subtract on the denominator). see section 1B.3.5
- Answer b
-
5.64 x 107 M bytes
\[(56.4Tbytes)(\frac{10^{12}bytes}{1 Tbytes})(\frac{1Mbytes}{10^{6}bytes})=56.4*10^{(12-6)}Mbytes=56.4*10^{6}Mbytes= 5.64*10^{7}Mbytes\]
Exercise \(\PageIndex{2}\)
Convert the following:
- 34.6 n sec to _____ G sec
- 4.0 Mg to _____ kg
- Answer a
-
3.46 x 10-17 G sec
- Answer b
-
4.0 x 103 kg
Exercise \(\PageIndex{3}\)
Convert the following:
- 3.57 x 1014 µL to _____ mL
- 4.337 x 1014 TL to _____ µL
- Answer a
-
3.57 x 1011 mL
\[(3.57*10^{14}\mu L)(\frac{10^{3}mL}{10^{6}\mu L})=3.57*10^{\left ( 14+3-6 \right )}\mu L=3.57*10^{11}\mu L\]
- Answer b
-
4.337 x 1032 µL
\[(4.337*10^{14} TL)(\frac{10^{6}\mu L}{10^{-12} TL})=4.337*10^{\left ( 14+6+12 \right )}\mu L=4.337*10^{32}\mu L\]
Exercise \(\PageIndex{4}\)
Convert the following:
- 4.78 x 1024 fg to _____ Mg
- 2.00 x 10-4 µsec to _____ psec
- Answer a
-
4.78 x 103 Mg
- Answer b
-
2.00 x 102 psec
Conversions Between Different Scales
Exercise \(\PageIndex{5}\)
Convert the following:
- 1.00 mL to _____ in3 (1 mL = 1 cm3)
- 1.00 TL to _____ ft3 (1 mL = 1 cm3)
- Answer a
-
0.0610 in3
\[(1.00 mL)(\frac{1 cm^{3}}{1 mL})(\frac{1 in}{2.54 cm})^{3}=0.0610 in^{3}\]
- Answer b
-
3.53 x 1010 ft3
\[(4.337*10^{14} TL)(\frac{10^{6}\mu L}{10^{-12} TL})=4.337*10^{\left ( 14+6+12 \right )}\mu L=4.337*10^{32}\mu L\]
Converting Floating Decimal Point Numbers to Scientific Notation
Exercise \(\PageIndex{6}\)
Convert the following to scientific notation:
- 0.00045602
- 50444000
- 253
- Answer a
-
4.5602 x 10-4
- Answer b
-
5.0444000 x 107
- Answer c
-
2.53 x 102
Exercise \(\PageIndex{7}\)
Convert the following to scientific notation:
- 50444000
- 32.010
- 0.0034
- Answer a
-
5.0444 x 107
- Answer b
-
3.2010 x 101
- Answer c
-
3.4 x 10-3
Mathematical Operations with Scientific Notation and Significant Figures
Exercise \(\PageIndex{8}\)
Solve the following problems:
- \begin{equation}
3.03860 \times 10^{32}+3.7 \times 10^{30}+3.68 \times 10^{31}
\end{equation} - \begin{equation}
3.03860 \times 10^{-32}+3.7 \times 10^{-30}+3.68 \times 10^{-31}
\end{equation}
- Answer a
-
\( \begin{align}\nonumber & 3.03860 \times 10^{32}\\\nonumber &0.037 \times 10^{32}\\\nonumber+& 0.368\times 10^{32}\\ \hline & 3.4436 \times 10^{32} \end{align}\)
The answer is \(3.444\times 10^{32}\) because two of the least precise value was known to the thousandths (section 1B3.4.2)
- Answer b
-
\( \begin{align}\nonumber & 3.7 \times 10^{-30}\\\nonumber &0.368 \times 10^{-30}\\\nonumber+& 0.0303860\times 10^{-30}\\ \hline & 4.098386\times 10^{-30} \end{align}\)
The answer is \(4.1\times 10^{-30}\) because two of the least precise value was known to the thousandths (section 1B3.4.2)
Exercise \(\PageIndex{9}\)
Solve the following problems:
- \begin{equation}
\frac{\left(9.7 \times 10^{583}\right)\left(7.339 \times 10^{98}\right)}{\left(5.432 \times 10^{-645}\right)\left(3.446 \times 10^{-4484}\right)}
\end{equation} - \begin{equation}
\frac{\left(9.7 \times 10^{583}\right)\left(7.339 \times 10^{-98}\right)}{\left(5.432 \times 10^{-645}\right)\left(3.446 \times 10^{4484}\right)}
\end{equation}
- Answer a
-
\(\frac{(9.7\times10^{583})(7.339\times10^{98})}{(5.432\times10^{-645})(3.446\times10^{-4484})}\\(\frac{9.7\times7.339}{5.432\times3.446})(10^{583+98-(-645)-(-4484)})\\3.80306\times10^{5810}\)
The answer is \(3.8\times 10^{5810}\) (section 1B3.4.2)
- Answer b
-
\(\frac{(9.7\times10^{583})(7.339\times10^{-98})}{(5.432\times10^{-645})(3.446\times10^{4484})}\\(\frac{9.7\times7.339}{5.432\times3.446})(10^{583-98-(-645)-(-4484)})\\3.80306\times10^{5614}\)
The answer is \(3.8\times 10^{5614}\) (section 1B3.4.2)
Exercise \(\PageIndex{10}\)
Solve the following problems:
- \begin{equation}
\frac{\left(9.7 \times 10^{-583}\right)\left(7.339 \times 10^{-98}\right)}{\left(5.432 \times 10^{645}\right)\left(3.446 \times 10^{4484}\right)}
\end{equation} - \begin{equation}
\frac{\left(9.70 \times 10^{5}+6.3 \times 10^{4}\right)\left(3.33 \times 10^{5}\right)}{\left(5.00 \times 10^{45}\right)\left(3.20 \times 10^{-5}\right)}
\end{equation}
- Answer a
-
\(\frac{(9.7\times10^{-583})(6.3\times10^{-98})}{(5.432\times10^{645})(3.446\times10^{4484})}\\(\frac{9.7\times7.339}{5.432\times3.446})(10^{-583-98-645-4484})\\3.80306\times10^{-5810}\)
The answer is \(3.8\times 10^{-5810}\) (section 1B3.4.2)
- Answer b
-
\(\frac{(9.70\times10^5\;+\;6.30\times10^4)(3.33\times10^5)}{(5.00\times10^{45})(3.20\times10^{-5})}\\\frac{(9.70\times10^5\;+\;0.63\times10^5)(3.33\times10^5)}{(5.00\times10^{45})(3.20\times10^{-5})}\\\frac{(10.33\times10^5)(3.33\times10^5)}{(5.00\times10^{45})(3.20\times10^{-5})}\\(\frac{10.33\times3.33}{5.00\times3.20})(10^{5+5-45-(-5)})\\2.1499\times10^{-30}\)
The answer is \(2.15\times 10^{-30}\) (section 1B3.4.2)
Conversions
Exercise \(\PageIndex{1}\)
To prepare for a laboratory period, a student lab assistant needs 125 g of a compound. A bottle containing 1/4 lb is available. Did the student have enough of the compound?
- Answer
-
\(1 \;lb\;=\;454\;g\\( \frac{1\;\textcolor{red}{ \cancel{lb}}}{4}) ( \frac{454 \; g}{1 \;\textcolor{red}{ \cancel{lb}}})=113\;g\)
No, 1/4 lb is only about 113 grams. .
Dimensional Analysis and Volume
Exercise \(\PageIndex{2}\)
Solve these problems about lumber dimensions.
- To describe to a European how houses are constructed in the US, the dimensions of “two-by-four” lumber must be converted into metric units. The thickness × width × length dimensions are 1.50 in. × 3.50 in. × 8.00 ft in the US. What are the dimensions in cm × cm × m?
- This lumber can be used as vertical studs, which are typically placed 16.0 in. apart. What is that distance in centimeters?
- Answer a
-
\(1 \;cm\;=\;2.54\;in\\1\;ft\;=\;.3048\;m\\1.50\textcolor{red}{ \cancel{in}}( \frac{2.54 \; cm}{1 \;\textcolor{red}{ \cancel{in}}})=3.81\;cm\\3.50\textcolor{red}{ \cancel{in}}( \frac{2.54 \; cm}{1 \;\textcolor{red}{ \cancel{in}}})=8.89\;cm\\8.00\textcolor{red}{ \cancel{ft}}( \frac{.3048 \; m}{1 \;\textcolor{red}{ \cancel{ft}}})=2.44\;m\\3.81\;cm\;\times\;8.89\;cm\;\times\;2.44\;m\)
The answer is 3.81 cm × 8.89 cm × 2.44 m (section 1B.4.2)
- Answer b
-
\(1 \;cm\;=\;2.54\;in\\16.0\textcolor{red}{ \cancel{in}}( \frac{2.54 \; cm}{1 \;\textcolor{red}{ \cancel{in}}})=40.6\;cm\)
The answer is 40.6 cm (section 1B.4.2)
Exercise \(\PageIndex{3}\)
Calculate these volumes.
- What is the volume of 11.3 g graphite in meters cubed, density = 2.25 g/cm3?
- What is the volume of 39.657 g bromine in liters, density = 2.928 g/cm3?
- Answer a
-
\(D=\frac{m}{V} \\ V=\frac{m}{D}\\V=\frac{11.3g}{2.25\frac{g}{cm^3}}\\V=5.02 cm^3\\V=\left (5.02 cm^3 \right )\left ( \frac{1m}{100cm^3} \right )^{3}=5.02\times 10^{-6}\;m\)
5.02 × 10-6 m3 (section 1B.4.2)
- Answer b
- \(D=\frac{m}{V} \\ V=\frac{m}{D}\\V=\frac{39.657g}{2.928\frac{g}{cm^3}}\\V=13.54 cm^3\\V=\left (13.54 cm^3 \right )\left ( \frac{1L}{1000cm^3} \right )^{3}=5.02\times 10^{-2}\;L\)
- 1.354 ×10-2 L (section 1B.4.2)
Temperature Conversions
Exercise \(\PageIndex{1}\)
Convert the following:
- 37.0 ºC to _____ K
- 331 K to _____ ºC
- Answer a
-
310.2 K
\[(37.0+273.15)=310.2K\]
(see section 1B.1.5)
- Answer b
-
57.9 ºC
\begin{equation}
(331-273.15)=57.9^{\circ} \mathrm{C}
\end{equation}(see section 1B.1.5)
Exercise \(\PageIndex{2}\)
Convert the following:
- 57.8 ºC to _____ ºF
- -129 ºF to _____ ºC
- Answer a
-
136 ºF
\begin{equation}
[(57.8+40) 1.8]-40=136^{\circ} \mathrm{F}
\end{equation}(see section 1B.1.5)
- Answer b
-
-89.4 ºC
\begin{equation}
\left(\frac{(-129+40)}{1.8}\right)-40=-89.4^{\circ} \mathrm{C}
\end{equation}(see section 1B.1.5)
Exercise \(\PageIndex{3}\)
Convert the boiling temperature of gold, 2966 °C, into degrees Fahrenheit and Kelvin.
- Answer
-
\(\left [\left (2966^{\circ}C+40 \right ) 1.8 \right ]-40=5371^{\circ}F\)
\(2966 ^{\circ} C +273.15 = 3239K\)
(see section 1B.1.5)
Exercise \(\PageIndex{4}\)
Convert the temperature of scalding water, 54 °C, into degrees Fahrenheit and Kelvin.
- Answer
-
\(\left [\left (54^{\circ}C+40 \right ) 1.8 \right ]-40=130^{\circ}F\)
\(54 ^{\circ} C +273.15 = 330\)
130 °F, 330 K
(see section 1B.1.5)
Exercise \(\PageIndex{5}\)
Convert the temperature of the coldest area in a freezer, −10 °F, to degrees Celsius and Kelvin.
- Answer
-
\( [(\frac{10 ^\circ F\;+\;40)}{1.6}]-40\;=\;-23 ^{\circ} C \)
\( [(\frac{10 ^\circ F\;+\;40)}{1.6}]-40\;+\;273.15\;=\;250\;K\)
−23 °C, 250 K
(see section 1B.1.5)
Algebra Review
Exercise \(\PageIndex{1}\)
Solve For \(A\)
\(A(X+Y)=Z\)
- Answer
- \(A(X+Y)=Z\\\frac{A(X+Y)}{X+Y}=\frac{Z}{X+Y}\\\frac{A\cancel{(X+Y)}}{\cancel{X+Y}}=\frac{Z}{X+Y}\\A=\frac{Z}{X+Y}\)
Exercise \(\PageIndex{1}\)
Solve For \(A\)
\((AX+Y)=Z\)
- Answer
-
\((AX+Y)=Z\\(AX+Y)-Y=Z-Y\\(AX+\cancel{Y})-\cancel{Y}=Z-Y\\\frac{AX}{X}=\frac{Z-Y}{X}\\\frac{A\cancel{X}}{\cancel{X}}=\frac{Z-Y}{X}\\A=\frac{Z-Y}{X}\)
Exercise \(\PageIndex{1}\)
Solve For \(A\)
\((AX+AY)=Z\)
- Answer
-
\((AX+AY)=Z\\A(X+Y)=Z\\\frac{A(X+Y)}{(X+Y)}=\frac{Z}{X+Y}\\\frac{A\cancel{(X+Y)}}{\cancel{(X+Y)}}=\frac{Z}{X+Y}\\A=\frac{Z}{X+Y}\)
Exercise \(\PageIndex{1}\)
Solve For \(A\)
\((AX+Y)Z=1\)
- Answer
-
\((AX+Y)Z=1\\\frac{(AX+Y)Z}{Z}=\frac{1}{Z}\\\frac{(AX+Y)\cancel{Z}}{\cancel{Z}}=\frac{1}{Z}\\AX+Y-Y=\frac{1}{Z}-Y\\AX+\cancel{Y}-\cancel{Y}=\frac{1}{Z}-Y\\\frac{AX}{X}=\frac{\frac{1}{Z}-Y}{X}\\\frac{A\cancel{X}}{\cancel{X}}=\frac{\frac{1}{Z}-Y}{X}\\A=\frac{\frac{1}{Z}-Y}{X}\)
Exercise \(\PageIndex{1}\)
Solve For \(A\)
\(\frac{A}{X+Y}=Z\)
- Answer
-
\(\frac{A}{X+Y}=Z\\\frac{A}{X+Y}(X+Y)=Z(X+Y)\\\frac{A}{\cancel{X+Y}}\cancel{(X+Y)}=Z(X+Y)\\A=Z(X+Y)\)
Exercise \(\PageIndex{1}\)
Solve For \(A\)
\(\frac{1}{AX+AY}=Z\)
- Answer
-
\(\frac{1}{AX+AY}=Z\\AX+AY=\frac{1}{Z}\\A(X+Y)=\frac{1}{Z}\\\frac{A(X+Y)}{(X+Y)}=\frac{1}{Z(X+Y)}\\\frac{A\cancel{(X+Y)}}{\cancel{(X+Y)}}=\frac{1}{Z(X+Y)}\\A=\frac{1}{Z(X+Y)}\)
Exercise \(\PageIndex{1}\)
Solve For \(A\)
\(\frac{1}{X+AY}=Z\)
- Answer
-
\(\frac{1}{X+AY}=Z\\X+AY=\frac{1}{Z}\\X+AY-X=\frac{1}{Z}-X\\\cancel{X}+AY\cancel{-X}=\frac{1}{Z}-X\\\frac{AY}{Y}=\frac{\frac{1}{Z}-X}{Y}\\\frac{A\cancel{Y}}{\cancel{Y}}=\frac{\frac{1}{Z}-X}{Y}\\A=\frac{\frac{1}{Z}-X}{Y}\)
Exercise \(\PageIndex{1}\)
Solve For \(A\)
\(\frac{A}{X+Y}=ZA+3\)
- Answer
-
\(\frac{A}{X+Y}=ZA+3\\\frac{A}{X+Y}(X+Y)=(ZA+3)(X+Y)\\\frac{A}{\cancel{(X+Y)}}\cancel{(X+Y)}=(ZA+3)(X+Y)\\A=ZA(X+Y)+3(X+Y)\\A-ZA(X+Y)=ZA(X+Y)-ZA(X+Y)+3(X+Y)\\A-ZA(X+Y)=\cancel{ZA(X+Y)}-\cancel{ZA(X+Y)}+3(X+Y)\\ A(1-ZA(X+Y))=3(X+Y)\\\frac{A(1-ZA(X+Y))}{(1-ZA(X+Y))}=\frac{3(X+Y)}{(1-ZA(X+Y))}\\\frac{A\cancel{(1-ZA(X+Y))}}{\cancel{(1-ZA(X+Y))}}=\frac{3(X+Y)}{(1-ZA(X+Y))}\\A=\frac{3(X+Y)}{1-Z(X+Y)}\)
Exercise \(\PageIndex{1}\)
Solve For \(A\)
\(\frac{A}{X+Y}=ZA+D\)
- Answer
-
\(\frac{A}{X+Y}=ZA+D\\\frac{A}{X+Y}(X+Y)=(ZA+D)(X+Y)\\\frac{A}{\cancel{(X+Y)}}\cancel{(X+Y)}=(ZA+D)(X+Y)\\A=ZA(X+Y)+D(X+Y)\\A-ZA(X+Y)=ZA(X+Y)-ZA(X+Y)+D(X+Y)\\A-ZA(X+Y)=\cancel{ZA(X+Y)}-\cancel{ZA(X+Y)}+D(X+Y)\\ A(1-ZA(X+Y))=D(X+Y)\\\frac{A(1-ZA(X+Y))}{(1-ZA(X+Y))}=\frac{D(X+Y)}{(1-ZA(X+Y))}\\\frac{A\cancel{(1-ZA(X+Y))}}{\cancel{(1-ZA(X+Y))}}=\frac{D(X+Y)}{(1-ZA(X+Y))}\\A=\frac{D(X+Y)}{1-Z(X+Y)}\)
Exercise \(\PageIndex{1}\)
Solve For \(A\)
\(\frac{A+P}{X+Y}=ZA+D\)
- Answer
-
\(\frac{A+P}{X+Y}=ZA+D\\\frac{A+P}{X+Y}(X+Y)=(ZA+D)(X+Y)\\\frac{A+P}{\cancel{(X+Y)}}\cancel{(X+Y)}=(ZA+D)(X+Y)\\A+P=ZA(X+Y)+D(X+Y)\\A+P-P=ZA(X+Y)+D(X+Y)-P\\A+\cancel{P}-\cancel{P}=ZA(X+Y)+D(X+Y)-P\\A-ZA(X+Y)=ZA(X+Y)-ZA(X+Y)+D(X+Y)-P\\A-ZA(X+Y)=\cancel{ZA(X+Y)}-\cancel{ZA(X+Y)}+D(X+Y)-P\\ A(1-ZA(X+Y))=D(X+Y)-P\\\frac{A(1-ZA(X+Y))}{(1-ZA(X+Y))}=\frac{D(X+Y)-P}{(1-ZA(X+Y))}\\\frac{A\cancel{(1-ZA(X+Y))}}{\cancel{(1-ZA(X+Y))}}=\frac{D(X+Y)-P}{(1-ZA(X+Y))}\\A=\frac{D(X+Y)-P}{1-Z(X+Y)}\)

