1B.1: Units of Measurement
- Page ID
- 158397
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Recognize different ways to quantity matter (measured, exact, ande defined numbers)
- Recognize English Units and Metric Units and SI units
- Understand the evolution of the definitions of units over time and their uses in society
- Convert between different units
Quantification of Matter
Scientists use three basic types of numbers when quantifying matter; counted numbers, measured numbers and defined numbers.
Counted Numbers: A counted number is an exact number in the sense that you have an entity, and you count the number of entities. So an exact number has two parts, the entity, and the number of entities. If you have 16 rocks in your hand, you have sixteen rocks, not more, not less. If you break one of the rocks into two, you now have 17 rocks in your hand, not more, not less. These are exact numbers. Counted numbers are exact and there is no uncertainty about their value.
Measured Numbers: Now the mass of the rocks is a measured number and requires a third aspect to describe, which is the unit, defined by the scale used to measure it. So a measured number has 3 parts; magnitude, unit and entity. Sixteen one pound rocks weighs less than one 500 pound rock, although the number 16 is more than the number one, and this is because different units are being used to describe the mass. If you break each of the sixteen one pound rocks in half, you have 32 rocks, but they still weigh the same. Measured numbers have uncertainty that is indicated by their number of significant digits (section 1B.2.2).
Defined Numbers: A defined number has a value inherent in its definition, and like a counted number is an exact number, but it may, or may not have uncertainty. Defined numbers are often used in measurements, for example, twelve inches is defined to be one foot and there is no uncertainty in that equivalence.
\[12 \; inches \equiv 1 \;foot \nonumber\]
But not all defined numbers are integers, like the number \(\pi\), which is defined to be ratio of the circumference to the diameter of a circle.
\[\pi \equiv \frac{circumference}{diameter} \nonumber \]
\(\pi\) is an exact number in the sense that it is exactly the ratio of the circumference to the diameter of a circle, but it is an irrational number and like a measured number, when we write down the value of \(\pi\) we give it a precision (3.14, 3.142, 3.1416, 3.14159,...), which defines how precisely we express the number.
Understanding defined numbers is of importance, and as we shall learn, as of May 20, 2019, the basic units of the metric system became based on defined numbers (2019 SI unit definitions).
Metrology
Metrology is the science of measurement. There are two fundamental tenets to metrology, traceability and uncertainty. Traceability is the ability to relate a measurement to a standard through calibrations, where each calibration has an element of uncertainty, which relates to how precisely the measurement (calibration) was made (see section 1B.2). Inherent in a standard are the definition upon which it is based, and the "method" used to put this definition into practice, which is often known by the French term "mise en pratique". In modern metrology the "mise en pratique" is a set of instructions that allows implementation of the standard into the practice of measurement, and results in the traceability and uncertainty of a measurement to the standard. The earliest standards were related to physical objects, and from these, tools like a ruler could be created that allowed the measurement of other objects in the unit of the standard. Early units included body parts, like the foot, which varied from person to person, and so a standard foot was required. A multitude of units could describe the same measurement (see figure 1B1.1 for different ways to measure length).
 |
|
| Figure \(\PageIndex{1}\): On the left are some of the units to describe length, some of which were based on human body parts (foot) or actions (pace). | |
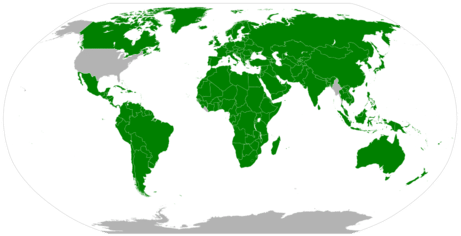
Today science uses the metric system. In fact most countries in the world use the metric system as the normal units of measurement.
|
|
| Figure \(\PageIndex{2}\): Areas of the world using the metric system. |
The customary units of measurement in the US are the foot, inch and pound, even though most of the rest of the world uses the metric system, as does the scientific community.
What may be surprising for most students is that a year after the civil war the U.S. congress passed the metric act, which made the metric system legal for purpose of commerce.
A bit of U.S. history
|
|
|
| Figure \(\PageIndex{3}\): U.S. Metric Act of 1866 that made the metric a legal unit in the U.S., even though today, the US is one of the few countries that does not use the metric system in normal commerce. | |
So what is the metric system?
Metric Units
Early Definitions
The Metric unit system evolved out of the French Revolutionary War, and was originally based on a unit of length, the meter. The earliest definition was the meridional definition, defining the meter as one ten-millionth of the distance from the north pole to the equator along the meridian going through Paris. This was done by surveying the distance from a belfry in Dunkirk France to Montjuic castle in Barcelona Spain, which was assumed to be one tenth the length of the Paris meridian. While the meridional metre survey was being conducted a series of prototype platinum bars were developed, and in 1799 the one that was closest to the meridional meter was chosen to be the standard meter (Wikipedia: History of the Metre). It should be noted that the original metric system was based entirely on this length, where the unit of mass was the gram, which was defined as the mass of a cube of water at 4oC that was one hundredth of a meter on each side (1 cm3). As this unit was very small compared to items that would be used in commerce, a prototype artifact of pure platinum was created that weighed 1000g, which became the first standard kilogram. This lasted until 1875 and the Treaty of the Meter.
| Property | Unit | Symbol |
|---|---|---|
| length | meter | m |
| mass | gram | g |
| volume | liter | L |
Table \(\PageIndex{1}\): Early Metric Units.
Note, one of the advantages of the metric system is the decimalization of the magnitude of units. This allowed easy conversion from small to large scale measurements. To go from centimeters to kilometers you simply multiple by 10,000, which is much easier to do than to convert the similar English units of inches to miles (see section 1B.3.5 Unit Conversions).
Treaty of the Meter
On May 20, 1875 the US was one of 17 nations to sign the "Treaty of the Meter" (the Metric Convention), which established the "Bureau International des Poids et Mesures" (BIPM), that was tasked to develop an international unified system of weights and measures. Through the BIPM's ongoing "Conférence Générale des Poids et Mesures" (CGPM), the standards continue to be revised and redefined to the present time. In addition to defining the standards the BIPM is also responsible for producing the "mise en pratique," which are published online. As a result of the metric convention 31 prototype meter standards of platinum/iridium were built, and "National Prototype standard 27" was the U.S. reference standard for length until 1983. The mass standard was now an iridium/platinum alloy kept in a vault in Sèvres France, under conditions specified by the Treaty of the Meter.
 |
 |
| Figure \(\PageIndex{4}\): prototype meter No. 27 (Credit: NIST) and International Prototype Kilogram (Credit BIPM) | |
Over time the metric unit system for measurement evolved into the SI or International System of Units.
International System of Units
In 1921 during the 6th meeting of the CGPM the scope and responsibilities of the BIPM was extended into other fields of physics (like temperature and time). In 1960 during during the 11th meeting of the CGPM, the International System of Units was established, and the system now officially became known as the SI system for its French spelling, Système international d'unités. The SI units are commonly called the metric units, they are the units used in scientific publications and as we shall see, are continually being revised and redefined through meetings of the CGPM.
There are seven SI base units that underpin all SI measurements. Through proper combinations they can describe other measured phenomena, like volume and energy, whose units of liter and joule are classified as derived SI units (section 1B.3.3.2).
SI Base Units
The seven SI base units are listed in table 1B.1.2. These can be combined to form derived units that describe any measurable physical quantity. Thus by defining these seven SI base units, any physical measurement can be reproduced. In this class we will use the first 5 units extensively; the meter, kilogram, second, kelvin and mole (and general chemistry 2 will also use the ampere).
| length | meter | m |  |
| mass | kilogram | kg | |
| time | second | s | |
| temperature (absolute) | kelvin | K | |
| amount of substance | mole | mol | |
| electric current | ampere | A | |
| luminous intensity | cd | ||
| Table\(\PageIndex{2}\): Seven SI Base Units. General chemistry I (chem 1402) students need to know the units in red. | Figure\(\PageIndex{5}\): Logo of Bureau international des poids et mesures (BIPM). | ||
Note a kilogram is 1,000 g (SI prefixes section 1B.3.3.3), which is roughly the volume of one liter of water (with the liter being a derived unit for volume). The kilogram is the only SI base unit with an SI prefix.
A few special points about some of the SI base units are worth noting
- The base unit of mass is unique in that an SI Prefixe is built into it (k for 1,000); i.e., the base SI unit is not the gram, but the Kilogram. This historically occurred because a gram is a real small quantity, and did not represent the magnitude of mass used in normal daily activities or commerce.
- Although the kilogram has an SI prefix in the base unit, you do not use Si prefixes on the kg, but on the gram (109 g is a gigagram, not a megakilogram).
- The base unit of time is the second, and time is not expressed as a decimalized metric number. Numerous attempts to make it so have never garnered any success; we are still stuck with the 24:60:60 system that we inherited from ancient times. The ancient Egyptians of around 1500 BC invented the 12-hour day, and the 60:60 part is a remnant of the base-60 system that the Sumerians used for their astronomical calculations around 100 BCE.
- Of special interest to Chemistry is the mole, the base unit for expressing the quantity of matter. It is now defined as exactly 6.02214076 x 1023 entities of anything.
1960-2019 Definitions
The following SI base unit definitions are adapted from NIST Special Publication 330 as obtained from the website, NIST Reference on Constants, Units and Uncertainty.
| meter | The distance of length traveled by light in a vacuum over the time interval of 1/299,792,458 of a second. (Note the speed of light in a vacuum is 299,792,458m/sec |
| kilogram | The mass the international iridium/platinum prototype kept in Paris France. |
| second | The duration of 9,192,631,770 periods of radiation corresponding to transitions on the cesium 133 atom (atomic clock) |
| kelvin | 1/273.16 of the thermodynamic temperature of the triple point of water |
| mole | the number of atoms in 12 grams of carbon-12 (the symbol is mol) |
| ampere | constant current maintained by two straight parallel conductors of infinite length in a vacuum that are 1 meter apart and produce between them a force of 2x10-7N/m |
| candela | the luminous intensity of a source of monochromatic radiation in a direction with a of frequency 540x1012 hetz in that direction with a flux of 1/683 watt per steradian. |
Table \(\PageIndex{3}\): Pre 2019 SI base unit definitions as adopted from NIST Special Publication 330.
The definitions in table 1B.1.3 are based on both artifacts and constants/phenomena. For example, the meter was originally based on an artifact in France, but in 1983 was changed to being defined by the speed of light (c) in a vacuum. In 1967 the second became defined by the cesium-133 atomic clock. In 1971 the mole was defined by the number of atoms in 12 grams of carbon-12, which required a sample of carbon-12. In 1954 the kelvin was defined by the triple point of water (a concept covered in general chemistry 2, section 12.7.3), which requires a sample of water (that has variable isotopic composition, a topic that will be on the first exam, section 2.3). But throughout this time, the definition of the kilogram never changed, it was still based on the standard kilogram in France.
There are some problems with the above definitions, the most obvious of which is the artifact-based definition of the kilogram. At the time of the creation of the original standard there were also "witness" standards made of identical mass, many of which became national prototypes. Over the years they would on occasion have their masses verified, and were shown to be changing (fig. 1B.1.6). The problem is, if a witness standard is getting "heavier", no one really know if it is really heavier, or if the prototype was getting lighter.

Figure\(\PageIndex{6}\) : Graph showing relative changes in mass of the witness BIPM prototype Kilogram artifacts relative to the International Prototype Kilogram (NIST)
2019 Definitions
On May 20, 2019 the definitions of the SI Base units were changed, and instead of defining 7 base units, seven constants that are considered to be invariants of nature are defined as exact numbers with no uncertainty. These constants are defined numbers, and this is a fundamental change as what is being defined are constants of nature and not the base units. The base unit is then obtained by applying the constant to the "mise en pratique". To do this, scientists first had to be able to measure these constants very accurately, and once done, they then defined the constant based on the measurement as an exact (defined) number. Then to apply it they sort of reverse engineered the process of measuring the constant, with the constant now be treated as a defined (exact) number, and could now measure the value of a sample based on the defined constant.
This sounds sort of complicated, but the process is actually easy to understand if we look at a constant we are familiar with, like density, the ratio of the mass of a substance to its volume. For an incompressible substance like water the density can be considered a constant, and if you define the density of water to be 1 g/ml as an exact definition, and have a device to measure volume, you can then use that to determine the mass. So the mass is determined by the defined value of the density as 1 g/mL (an exact number by definition). Of course the density of water as a constant is not an invariant of nature, and changes as temperature, pressure and other factors change. But the principle is the same.

Figure\(\PageIndex{7}\): Card with SI defining constants (Stoughton/NIST). These values are considered as defined numbers.
Many of these constants will be encountered as we proceed through the material of this class and although students need to know the SI base units, they do not need to be concerned with deriving the SI base units from the 7 defined constants.
It is hoped that by the time this chapter is finished students can understand the logic of the new definitions, and use units in their calculations. The key to this is that the constants must have the units of the base unit that they describe (density has units of mass over volume, so could be used to describe units of mass or volume). When you look at the card in figure 1B.1.7 you see units like J/Hz for Planck's constant. Those are actually SI derived units (section 1B.3.2), and Planck's constant can be described in units of \(\frac{kg\cdot m^{2}}{s}\), and so could be used to define the SI base units of Kilogram, meter and second. In sections 1B.3 Mathematics in Chemistry and section 1B.4 Dimensional Analysis, students will learn how to use units in calculations, and these type of math skills are what is required to mathematically relate the fundamental constants to the SI base units.
optional: deeper look
To get a better understanding of the change in definitions it may be prudent to look at the respective values of the new and old definitions. This information is obtained from The International System of Units, 8th ed. Section 2.1.1, BIPM (old definitions) and Appendix 3, Resolution 1 of 26th CGPM (new definitions).
Meter
- Previous definition: The metre is the length of the path travelled by light in vacuum during a time interval of 1/299,792,458 of a second
- 2019 definition: The metre, is defined by taking the fixed numerical value of the speed of light in vacuum c to be 299,792,458 when expressed in the unit m/s, where the second is defined in terms of ΔνCs
Kilogram
- Previous definition: The kilogram is the unit of mass; it is equal to the mass of the international prototype of the kilogram
- 2019 definition: The kilogram is defined by taking the fixed numerical value of the Planck constant h to be 6.626,070 15 × 10–34 when expressed in the unit J⋅s, which is equal to kg⋅m2⋅s–1, where the metre and the second are defined in terms of c and ΔνCs.
Second
- Previous definition: The second is the duration of 9,192,631,770 periods of the radiation corresponding to the transition between the two hyperfine levels of the ground state of the cesium 133 atom.
- 2019 definition: The second is defined by taking the fixed numerical value of the cesium frequency ΔνCs , the unperturbed ground-state hyperfine transition frequency of the cesium 133 atom, to be 9,192,631,770 when expressed in the unit Hz, which is equal to s–1.
Ampere
- Previous definition: The ampere is that constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross-section, and placed 1 metre apart in vacuum, would produce between these conductors a force equal to 2 × 10−7 newton per metre of length.
- 2019 definition: The ampere is defined by taking the fixed numerical value of the elementary charge e to be 1.602,176,634 × 10–19 when expressed in the unit C, which is equal to A s, where the second is defined in terms of ΔνCs.
Kelvin
- Previous definition: The kelvin, unit of thermodynamic temperature, is the fraction 1/273.16 of the thermodynamic temperature of the triple point of water.
- 2019 definition: The kelvin is defined by taking the fixed numerical value of the Boltzmann constant k to be 1.380649 × 10–23 when expressed in the unit J⋅K–1, which is equal to kg⋅m2⋅s–2⋅K–1, where the kilogram, metre and second are defined in terms of h, c and ΔνCs.
Mole
- Previous definition: The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon 12.
- 2019 definition:.The mole is the SI unit of amount of substance and contains exactly 6.02214076 × 1023 elementary entities. This number is the fixed numerical value of the Avogadro constant, NA,
Candela
- Previous definition: The candela is the luminous intensity, in a given direction, of a source that emits monochromatic radiation of frequency 540 × 1012 hertz and that has a radiant intensity in that direction of 1/683 watt per steradian
- 2019 definition: The candela is defined by taking the fixed numerical value of the luminous efficacy of monochromatic radiation of frequency 540×1012 Hz, Kcd, to be 683 when expressed in the unit lm⋅W−1, which is equal to cd⋅sr⋅W−1, or cd⋅sr⋅kg−1⋅m−2⋅s3, where the kilogram, metre and second are defined in terms of h, c and ΔνCs.
The kilogram is probably the unit whose definition has undergone the most radical change as it was the only previous definition that depended on one specific artifact, and now involves the use a a device called the Watt balance. The Watt balance essentially balances gravitational forces with electromagnetic to determine Planck's constant. Once Planck's constant was determined, new "secondary standard" kilograms can be created by sort of reverse engineering the use of the device, but even this is very complicated, as the Watt balance operates in a vacuum and cryogenic temperatures, and the secondary standard needs to be in atmospheric conditions where normal measurements occur. The article "A LEGO Watt balance: An apparatus to determine a mass based on the new SI", gives a pretty good walk though on how the Watt balance is used.
Students who wish to learn more about the new SI definitions are encouraged to look at the resources provided through NIST's SI Redefintion website. The American Scientist article "Weighing the Kilogram" by Paul Karol is also an easy to read article going over many of the issues associated with defining the kilogram and the mole.
Note: All students need to know what the SI Base units are, and if they see the definitions of the base units on an exam, they need to determine if they relate to the pre or post May 2019 revisions, as many textbooks and exam banks will still be using the old ones.
SI Derived Units
In the previous section we stated that the SI base units could be combined to measure different types of physical phenomena. Figure 1B.1.8 shows common units of measure that can be derived from the base units. There are two types, those that do not have special SI names and symbols, like area (m2), and those that do, like pressure, work,... So for example, the SI unit of energy is the Joule, which is a "derived unit" and can be described in terms of the base units of mass, length and time:
\[\underbrace{1J=1\frac{kg\cdot m^{2}}{s^{2}}}_{\text{SI Derived Unit of Energy in terms of SI Base Units}} \]
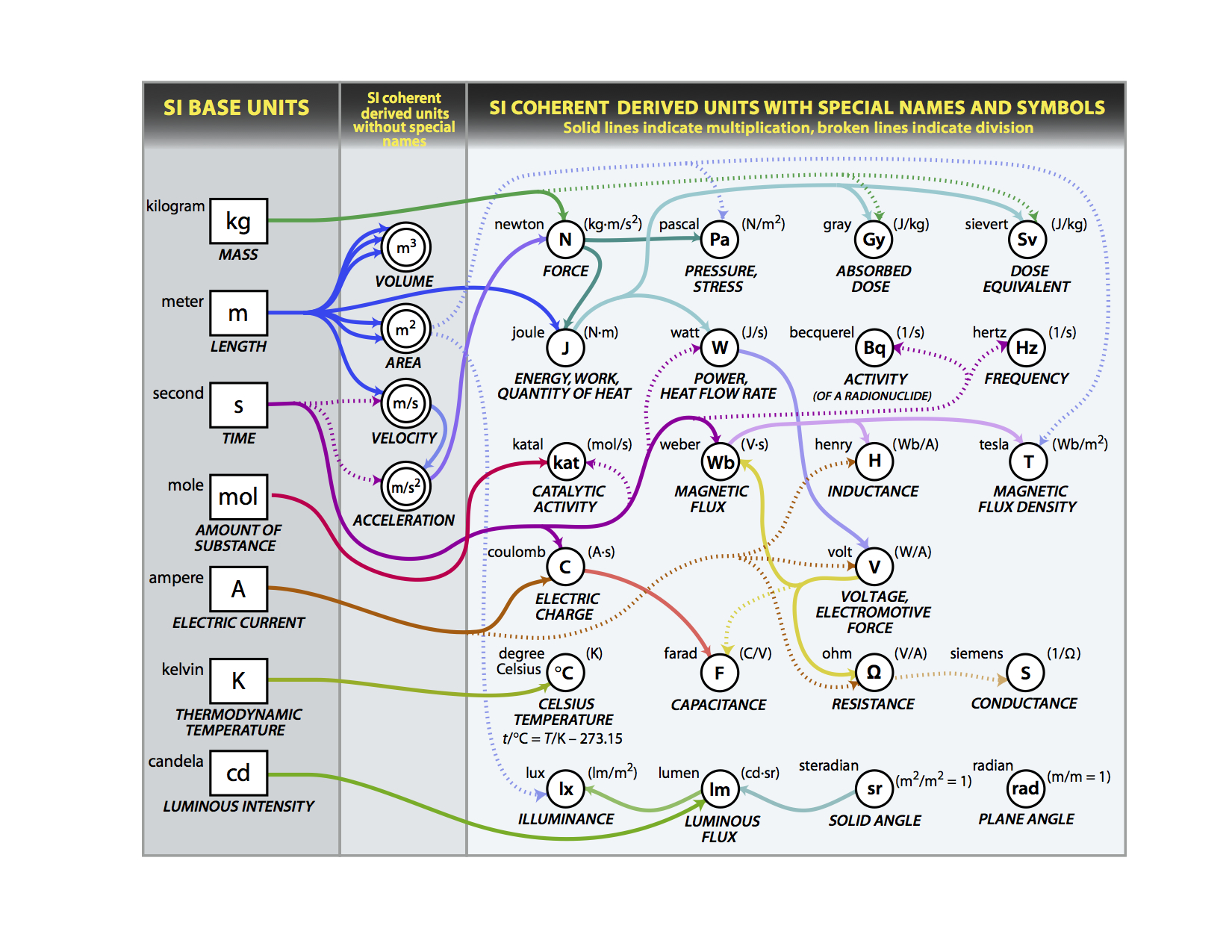
Figure\(\PageIndex{8}\): Flowchart showing how different derived SI units can be created from the base units (NIST).
Table represents common SI units:
| Quantity | Unit | Explanation |
|---|---|---|
| Work | N | Newton = kg m s-2 |
| Pressure | Pa | Pascal = N m-2 |
| Energy | J | Joule = N.m |
| Charge | C | Coulomb = A.s |
| Electric Potential | V | Volt = J/C |
| Power | Watt | 1 watt = 1 J/s |
| Volume | L | 1L = 1dm3 |
| Volume | mL | 1mL = 1cm3 |
| Molarity | M | mol/L |
Table\(\PageIndex{4}\): Derivied SI units and their dependencies on the SI bas units.
Note, there are many non-SI units like the calorie, atm, foot, etc., which can also be described in terms of the SI base units. Conversions between units is covered in section 1B.3.5.
SI Prefixes
SI prefixs are commonly used to represent very large and small numbers. You need to memorize many of these and know how to convert between them.
| Multiple | Name | Abbreviation | Name | Multiple | ||
|---|---|---|---|---|---|---|
| 10+24 | yotta | Y | y | yocto | 10-24 | |
| 10+21 | zetta | Z | z | zepto | 10-21 | |
| 10+18 | exa | E | a | atto | 10-18 | |
| 10+15 | peta | P | f | femto | 10-15 | |
| 10+12 | tera | T | p | pico | 10-12 | |
| 10+9 | giga | G | n | nano | 10-9 | |
| 10+6 | mega | M | m | micro | 10-6 | |
| 10+3 | kilo | k | m | milli | 10-3 | |
| 10+2 | hecto | h | c | centi | 10-2 | |
| 10+1 | deca | da | d | deci | 10-1 | |
Table\(\PageIndex{5}\): SI prefixes. Note Chem 1402 students need to memorize all the values in red.
Use of SI prefixes
For real large or small numbers, the convention is to place a number with between 1 and 999 in front of the SI prefix, and use the appropriate prefix to show the value. So a memory stick with 1,200,000 bytes would be written as 1.2 Mbyte, not as 1,200 kbytes.
What is the ENG key on scientific calculators?
Figure\(\PageIndex{8}\): Three display modes on TI 30 calculator.
The ENG key takes large or small numbers and expresses them as integer multiples of 103 or 10-3, effectively converting them to values that can be expressed with SI prefixes. If you type 1,200,000 into your calculator and press ENG, it gives 1.2 x 106, so 1,200,000 bytes of memory on a memory stick would be 1.2 MBytes (you do not say 1,200 kBytes). If you try 0.12 and press ENG, it gives 120 x 10-3, so 0.12g is 120 mg. So the ENG function quickly converts numbers to values that can easily be expressed with SI prefixes.
In section 1B.3 we will go over mathematical operations using SI prefixes, and the conversions of units.
Vocabulary
Metric - decimal based unit of measure originally based on the meter, but evolved into SI.
Metrology - Science of Measurement
SI Base Unit - seven base units that all physical measurements can be derived from.
SI Derived Unit - a unit of measurment created by combining SI Base Units.
SI Prefixes - used to describe large or small values of SI units, usually of multiples of 103.
SI Unit - System international, unit of measure started in 1960 and evolving through the "Bureau International des Poids et Mesures" (BIPM), which was established by the Metric Convention of 1875. It is the established unit of measure for the practice of science.
Contributors
Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The breadth, depth and veracity of this work is the responsibility of Robert E. Belford, rebelford@ualr.edu. You should contact him if you have any concerns. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to: