18: Metabolism and Bioenergetics: Questions
- Page ID
- 43933
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Carbohydrate Catabolism
(01) Some people cannot digest the sugar lactose (lactose intolerant). Use the lactose molecule shown below to answer the following questions.

- What s the name of the enzyme tha would catalyze lactose digestion? What type of reaction is catalyzed - hydration, redox, synthetic dehydration, hydrolysis, or isomerzation?
- Classify lactose as a mono-, di-, oligo-, or polysaccharide/
- Circle the anomeric carbon.
- Point an arrow to the glycosidic bond and classify it using the alpha or beta-(#,#) format.
- If the glycosidic bond was broken, what are the names of the products? Remember to include the alpha or beta designator for the anomeric carbons.
(02) List the stages of catabolism that convert a polysaccharide into ATP. What are the major reactions, intermediate compounds formed, and biochemical pathways that occur?
(03) For which types of cells is glycolysis for sole source of energy? Name one advantage and one disadvantage when a cell relies solely on glycolysis for energy?
(04) Pyruvate can undergo different processes depending upon the conditions it can be found in. What happens to pyruvate when there is no oxygen to draw upon? What happens when there is oxygen available? Which of these two does not generate something that can proceed to the next stage of metabolism?
(05) Summarize the importance of carbohydrates, in the biochemical sense, using only one sentence.
(06) The process of glycolysis generates many products and requires numerous enzymes and other molecules to generate the various phases leading up to the final products. Which steps require the breakdown of ATP to ADP? Which steps involve building up ADP into ATP?
(07) Several steps in glycolysis involve a transfer reaction, and some involve multiple processes. Examine the diagram of phosphoenolpyruvate becoming pyruvate and describe, in words, all the changes that occur during this step.

(08) The catabolism of glucose can be divided into 3 stages (not steps). What is the end product of the first stage? What is the end product of the second stage? What is the name of the biochemical cycle that begins the third stage?
Fatty Acid Catabolism
(09) List the stages of catabolism that convert a triglyceride into ATP. What are the major reactions, intermediate compounds formed, and biochemical pathways that occur?
(10) Explain the process for the body obtaining fatty acids from our diets. What molecules are responsible for delivering fatty acids into the body? Are fatty acids ever consumed directly or only as part of large biomolecules?
(11) Explain why lipoproteins are needed to transport triglycerides in aqueous body fluids. Name the 5 major lipoproteins and explain how they are similar and how they are different.
(12) Lipoproteins have a membrane structure similar to that of cell membranes. Which portion, the exterior and interior, are polar and which are non-polar? Which lipoproteins have the greater densities, those with more protein in their composition or less protein?
(13) Cells contain many organelles with many different purposes and functions. Which organelle is responsible for beta-oxidation?
(14) How many steps are involved in beta-oxidation? What type of reaction does each step entail? Are any of them more frequent than others?
Acetyl Coenzyme A and the Citric Acid Cycle
(15) The body requires reactions that both build up smaller molecules into larger ones and break down larger ones into smaller ones. Which of the two processes is called anabolic, and which is called catabolic?
(16) From the processes discussed in this chapter, is acetyl CoA produced from anabolism or catabolism? Are any of the three major dietary biomolecules, i.e. proteins, carbohydrates and lipids, unable to contribute to the production of acetyl CoA?
(17) Examine the structure of acetyl CoA shown below. Then answer the following questions.
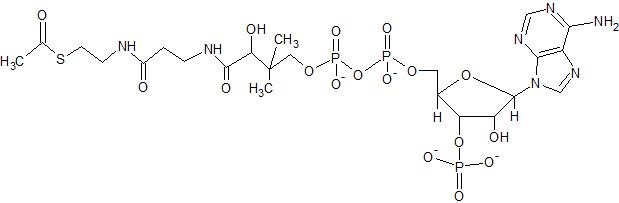
- Identify the acetyl group of acetyl CoA; what type of carbonyl group does it contain?
- Circle and identify all nitrogen-containing functional groups; make sure the labels for amines are able to be distinguished from the labels for amides
- Identify the phosphate groups and draw arrows pointing to any anhydride bonds
- For all other functional groups, indicate their locations with whatever type of marking you choose, and identify what type of functional group they belong to.
(18) An important component of the CAC is citrate, pictured below. Study the structure of citrate in order to answer the following.

- Based on the ionized state of citrate, is it likely at a low or high pH?
- Re-sketch citrate to show what it would look like at the appropriate pH to be fully protonated.
- Find the structure of isocitrate. Is isocitrate different in one way only, or more than one way? Circle and label all areas that are different between citrate and isocitrate.
- Look at the structures of citrate and isocitrate side by side. Do they fall under a more general category of isomers or can they be considered a more specific type of isomers?
(19) The first four steps of the CAC set citrate up for and then proceed to remove of carbonate groups; what compound do these groups exit as? Which oxidizing agent is involved in the steps leading up through step 4? How many are needed and become reduced by the end of step 4?
(20) From start to finish, how many molecules of NADH are produced in the CAC? How many molecules of FADH2are produced?
(21) Adenosine triphosphate (ATP) can be produced from NADH and FADH2 from the CAC. How many ATP molecules can NADH produce? How many can FADH2 produce?
(22) What particular two steps are responsible for releasing CO2? What is the name of the reaction that releases these molecules from their parent molecules?
Citric Acid Cycle
(23) The final step of the CAC is shown below. Use the information from the sketch to answer the following questions.

- What type of reaction does this step represent?
- Does L-malate get oxidized or reduced?
- Does NAD+ get oxidized or reduced?
(24) Examine step 7 of the CAC, pictured below, and answer the following questions.

- What type of reaction takes place during this step?
- Are there any chiral carbons present in the reactant or product?
(25) The initial step of the CAC binds oxaloacetate and acetyl CoA. Use the structures provided below, answer the questions that follow.

- What types of reactions happen to allow oxaloacetate and acetyl CoA to bond together?
- Show wherever cleavage occurs.
- Draw the product that forms from the reaction that will be used in step 2.
(26) Look at step 3 of the CAC, shown here. If the ∆H for the decarboxylation of alpha-ketoglutarate is negative, is the reaction considered exothermic or endothermic? On which side would we add the word "heat" - reactants or products?

Oxidative Phosphorylation
(27) What cellular organelles are responsible for facilitating energy production? Which specific energy-production pathways are handled by these organelles?
(28) Unlike most other processes discussed in this chapter, one type takes place in the cytoplasm; which process is this? What does this process do? Is it a catabolic or anabolic process?
(29) Like the cell itself, mitochondria have membranes as well. Is the inner or outer membrane of mitochondria the selectively permeable one?
(30) The proton gradient affects the concentration of H+ on each side; which side of the gradient would you expect to have a higher pH? Explain.
(31) What creates proton-motive force? How is this similar to a battery?
(32) What is the reason cyanide (CN-) ion is poisonous?
(33) Which components of the ETC are mobile electron carriers? Where in the cycle do they each operate?

