13: Reactions of Organic Functional Groups in Biochemistry: Questions
- Page ID
- 43927
Oxidation-Reduction (Redox) Reactions
(01) Think back to combustion reactions. Write the combustion reaction of CH4 and O2 and the products it forms; using the rules for determining oxidation numbers, determine which elements, of hydrogen, oxygen and carbon, undergo oxidation and reduction. Which undergoes oxidation? Which undergoes reduction?
(02) There are a few mnemonic devices for remembering the definition of oxidation and of reduction. Define the characteristic that makes a substance become oxidized and what makes a substance become reduced. What defines a substance as an oxidizing agent, and what defines it as a reducing agent?
(03) In terms of organic reactions, determine what effects the following operations have, whether oxidation or reduction.
- Losing bonds to hydrogen atoms
- Gaining bonds to oxygen atoms
- Gaining bonds to hydrogen atoms
- Losing bonds to oxygen atoms
(04) For the following equations, find the oxidation and reduction numbers of each element to determine which compound is oxidized and which is reduced and label them as such. Write the oxidation ½-reaction and the reduction ½-reaction.
- \(Al(s) + 3KOH(aq) \rightarrow 3K(s) + Al(OH)_{3\;(aq)}\)
- \(2H_{2\;(g)} + O_{2\;(g)} \rightarrow 2H_2O_{(g)}\)
- \(C_2H_{4\;(g)} + 3O_{2\;(g)} \rightarrow 2CO_{2\;(g)} + 2H_2O_{(g)}\)
- \(N_{2\;(g)} + 3H_{2\;(g)} \rightarrow 2NH_{3\;(g)}\)
- \(Cu(s) + 2HNO_{3\;(aq)} \rightarrow Cu(NO_3)_{2\;(aq)} + H_2(g)\)
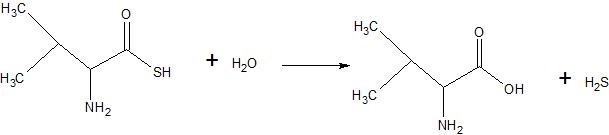
(05) Determine whether each compound below has undergone oxidation, reduction or neither.
a. 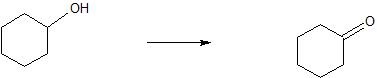
b. 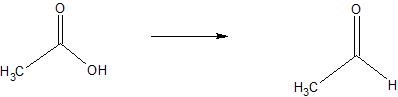
c. 
d. 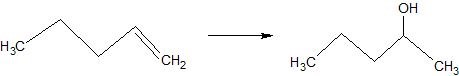
e. 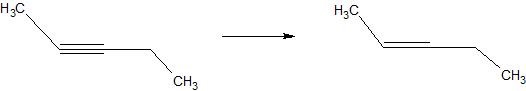
(06) For each coenzyme listed, classify it as an oxidizing or reducing agent.
- NADH + H+
- FAD
- NAD+
- FADH2
(07) For each of the following organic structures, show what the product would look like after a strong oxidation. If a reaction cannot take place, explain why.
a. 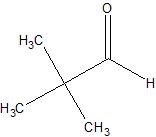
b. 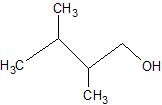
c. 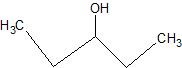
d. 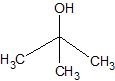
e. 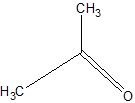
(08) For each of the following organic structures, draw the bond-line structure of the product after a complete (strong) reduction if a reduction reaction is possible.
a. 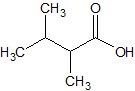
b. 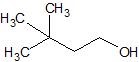
c. 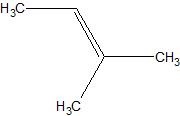
d. 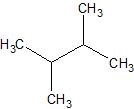
e. 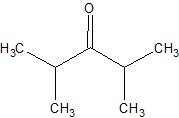
(09) The following are products of an oxidation or reduction reaction (there may be more than one possibility). Determine and sketch the structure of the reactant and tell whether it was oxidized or reduced.
a. 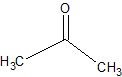
b. 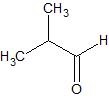
c. 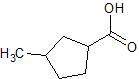
d. 3-methyl-2-butanol
e. butane
(10) Answer the following true or false questions regarding antioxidants.
- They can be found in many fruits and vegetables
- They are good oxidizing agents
- They stop the spread of free radicals in the body
- Many of their effects are not yet officially confirmed (such as slowing the aging process)
- They are good reducing agents
Hydration-Dehydration Reactions
(11) Determine whether each of the following reactions are hydration or dehydration. Use arrows to indicate whether water is being brought in or taken out.
a. 
b. 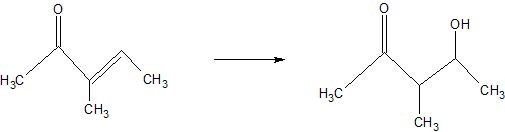
c. 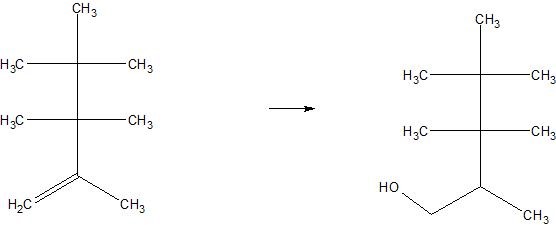
d. 
e. 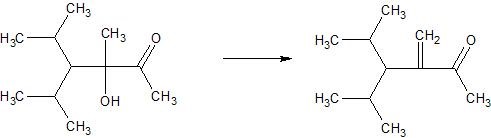
(12) Complete the following hydration and dehydration reactions and show what products form.
a. 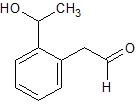
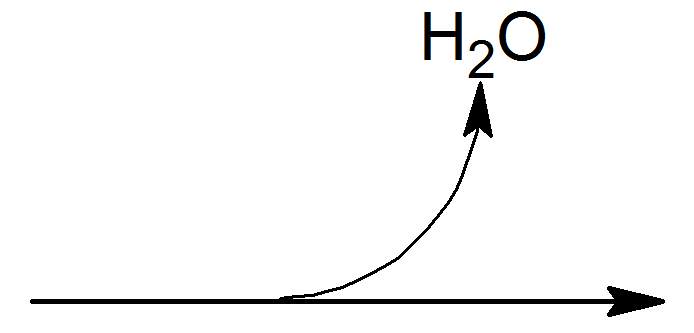
b. 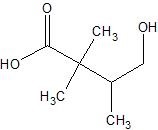

c. 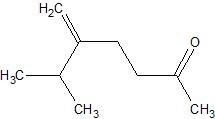
d. 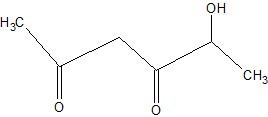

e. 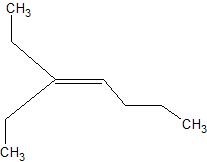
Acyl Group Transfer Reactions
(13) For the following enzyme catalyzed hydrolysis reactions at pH 7.4, determine the products.
a. 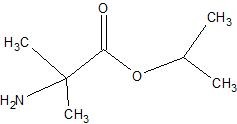
b. 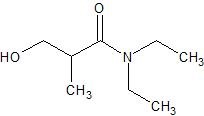
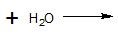
c. 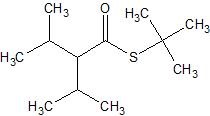
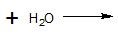
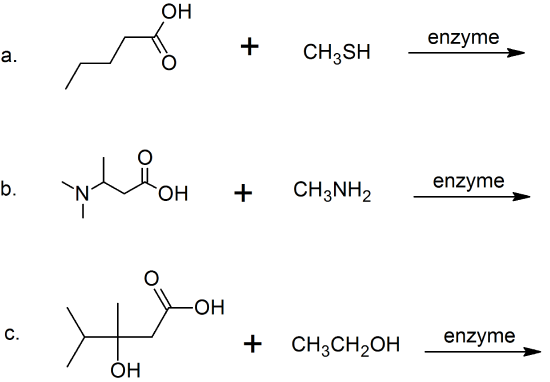
(14) For the following acyl transfer reactions, complete the equation by drawing the bond-line structures for the products.
(15) Determine the structure of the products for each esterification reaction.
a. 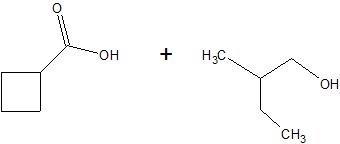
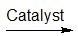
b. 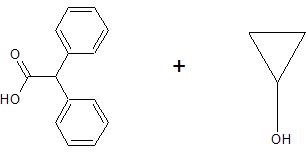
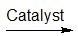
c. 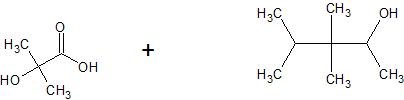
Phosphoryl Group Transfer Reactions
(16) ATP can undergo hydrolysis to transfer one of its phosphate groups. Use bond-line structures to write the reaction of ATP hydrolyzing to ADP.
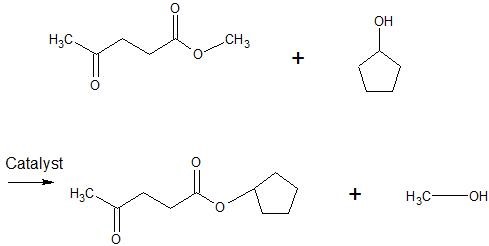
(17) Examine the following reactions and determine whether they are oxidation, reduction, hydration, dehydration, acyl transfer, or phosphoryl transfer.
a. 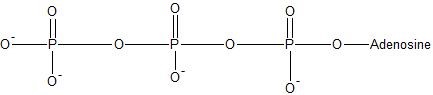
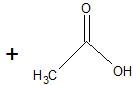
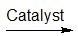
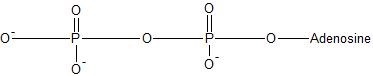
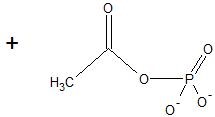
b. 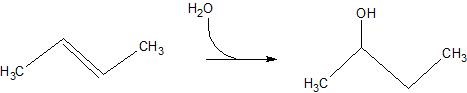
c.
. 
d. 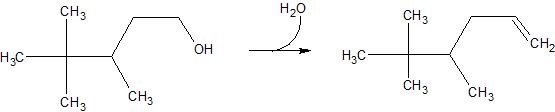
e.