Extra Credit 46
- Page ID
- 83443
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q19.25A
What are the ΔGo for the reactions below?
- Ti (s) + Cr2+ (aq)→Ti2+ (aq) + Cr (s)
- 2Cu+ (aq) + Sn4+ (aq)→2Cu2+ (aq) + Sn2+ (aq)
- 2Cl2 (g) + 2H2O (l)→O2 (g) + 4H+ (aq) + 4Cl- (aq)
S19.25A
We are asked to identify the Gibbs Free Energy (ΔGo) for each reaction. Our first step would be to analyze the equation and split the reaction into half-reactions. Our goal is obtaining an oxidation and a reduction equation to later use the half-reaction table and find the Eo values in Volts(V).

We know that a reduction half-reaction is a chemical species decreasing its oxidation number, usually by gaining electrons. The other half of the reaction involves oxidation, in which electrons are lost. Together, reduction and oxidation form redox reactions (reduction - oxidation = redox). A simple way to determine whether it is a reduction or oxidation equation is by the placement of the electrons. If the electrons are on the reactants side (behind the arrow), then it’s reduction, but it they are on the products side (what the arrow is pointing to), then it is oxidation.
1. From the overall equation, we can tell that Ti(s) and Ti2+(aq) are involved in the oxidation half-reaction. In order to balance the number of electrons on both sides, we need to add 2 electrons to the product side. We can then look at the half- reaction chart to determine the Eo of the half reaction. This brings us to:
Oxidation: Ti(s) →Ti2+(aq) + 2e- −Eo =+1.63V
The remaining elements will create our reduction half-reaction and be presented like:
Reduction: Cr2+ (aq) +2e- → Cr(s) Eo=−0.90V
If combined together, the overall net equation will be:
Net: Ti(s) + Cr2+(aq) → Ti2+(aq) + Cr(s) Eo=0.73V
From the half-reaction chart, we know that the value of our oxidation reaction must be reversed, thus presenting us with a negative Eo = +1.63V, and our reduction reaction is Eo = -0.90V. The overall net value can be found by Eo = E cell cathode – E cell anode or Eo = E cell reduction - E cell oxidation
Using this equation, we find that -0.90V – (-1.63V) = 0.73V.
Now, we take our Eo value and know that the Free-energy change, ΔGo, is related to cell potential, Eo, by the equation ΔGo=−nFEo, where n is the number of moles of electrons transferred and F=96,500 C /(mol e−) is the Faraday constant. When Eo is measured in volts, ΔGo must be in joules since 1 J=1 C⋅V.
ΔG∘=−nFEocell
= −(2 mole e-)(96,485 C/mol e-)(0.73 V)
= −140,868.1 J
Our answer is in Joules, but we need to change units into Kilojoules. Thus, we divide by 1000.
=−1.41×102 KJ.
2. From the overall equation, we can tell that Sn4+ (aq) and Sn2+(aq) are involved in the oxidation half-reaction. In order to balance the number of electrons on both sides, we need to add 2 electrons to the reactant side. This brings us to:
Oxidation: Sn2+(aq) → 2e- + Sn4+(aq) −Eo=−0.154V
The remaining elements will create our reduction half-reaction and be presented like:
Reduction: 2× (Cu2+(aq) + e- → Cu+ (aq)) Eo= 0.159 V
*We must multiply the entire equation by 2 because both the reduction and oxidation must have a balanced number of electrons on opposing sides.
If combined together, the overall net equation will be:
Net: 2 Cu+ (aq) + Sn4+ (aq) → 2 Cu2+(aq) + Sn2+(aq) Eocell=0.005V
From the half-reaction chart, we know that the value of our oxidation reaction must be reversed (negative), thus presenting us with a negative −Eo=−0.154V, and our reduction reaction is Eo = 0.159V. The overall net value can be found by Eo = E cell cathode – E cell anode, or Eo = E cell reduction - E cell oxidation
Using this equation, we find that 0.159V – (0.154V) = 0.005V.
Now, we take our Eo value and know that the Free-energy change, ΔGo, is related to cell potential, Eo, by the equation ΔGo=−nFEo, where n is the number of moles of electrons transferred and F=96,500 C /(mol e−) is the Faraday constant. When E∘ is measured in volts, ΔGEo must be in joules since 1 J=1 C⋅V.
ΔGo=−nFEocell
= −(2 mole e−)(96,485 C/mol e-)(0.005V)
=−946.85 J
Our answer is in Joules, but we need to change units into Kilojoules. Thus, we divide by 1000.
=−0.965 KJ
3. From the overall equation, we can tell that 2H2O (l), O2(g), and 4H+ (aq) are involved in the oxidation half-reaction. In order to balance the number of electrons on both sides, we need to add 4 electrons to the products side. This brings us to
Oxidation: 2 H2O(l) → O2(g) + 4H+ (aq) + 4e- −Eo=−1.229V
The remaining elements will create our reduction half-reaction and be presented like
Reduction: 2× (Cl2 (g) + 2e- → 2 Cl- (aq)) Eo=1.358V
*We must multiply the entire equation by 2 because both the reduction and oxidation must have a balanced number of electrons on opposing sides.
If combined together, the overall net equation will be
Net: 2 Cl2(g) + 2H2O(l) → O2(g) + 4H+(aq) + 4 Cl-(aq) Eocell=0.129V
From the half-reaction chart, we know that the value of our oxidation reaction must be reversed (negative), thus presenting us with a negative −Eo=−1.229V, and our reduction reaction is Eo = 1.358V. The overall net value can be found by Eo = E cell cathode – E cell anode, or Eo = E cell reduction - E cell oxidation
Using this equation, we find that 1.358V – (1.229V) = 0.129V.
Now, we take our Eo value and know that the Free-energy change, ΔGo, is related to cell potential, Eo, by the equation ΔGo=−nFEo, where n is the number of moles of electrons transferred and F=96,500 C /(mol e−) is the Faraday constant. When E∘ is measured in volts, ΔGo must be in joules since 1 J=1 C⋅V.
ΔGo=−nFEocell
=−(4 mole e-) (96,485 C/mol e-)(0.129V)
=−49,786.26J
Our answer is in Joules, but we need to change units into Kilojoules. Thus, we divide by 1000.
=−4.98×104 KJ.
Q20.5A
Find the electron potentials of reactions (a) and (b) of the diagram below.
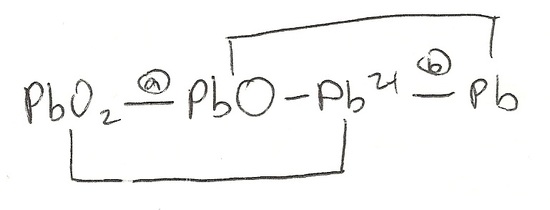
S20.5A
- PbO2(s) + 2H+(aq) + 2e_ → PbO(s) + H2O(l)
- Pb2+(aq) + 2e− → Pb(s)
The following are steps to help us reach our ultimately equation, but not all steps are needed; it varies from equation to equation. All steps are to be attempted in order from 1-8.
Step 1: Split reaction into half‐reactions (reduction and oxidation)
Step 2: Balance the charge or oxidation number with electrons
Step 3: Balance O by adding H2O
Step 4: Balance H by adding H+
Step 5: Multiply by some integer to make electrons (lost) = electrons (gained)
Step 6: Add half equations and cancel substances on both sides
Step 7: (only in basic solution): add OH- and cancel H2O
Step 8: Check atom balance and charge balance on both sides of the equation!
a. The diagram allows us to see that PbO2(s) transitions to PbO(s). With that we need to find how to get from point A to point B, in order to go from PbO2(s) to PbO(s).
Seeing what we have, we must first balance the oxygen by adding H2O, giving us:
PbO2(s) → PbO(s) + H2O(l)
Next we balance the hydrogen by adding H+ and get:
PbO2(s) + 2H+(aq) → PbO(s) + H2O(l)
*Notice there is a 2 in front of the H+ because water has 2 hydrogens.
In order to added electrons, we must first find the charges of each compound.
PbO2(s) has a charge of 0
2H+(aq) has a charge of +2
PbO(s) has a charge of 0
H2O(l) has a charge of 0
Meaning we must add 2 electrons to the reactants side to balance the overall charge of the reactants (2+) to that of the products (0), resulting in:
PbO2(s) + 2H+(aq) + 2e_ → PbO(s) + H2O(l)
b. This can be considered one of the simplest of cases because there are no other elements involved except for Pb (Pb2+ and Pb). We simply add 2 e- to the reactants to balance out the 2+ charge of the Pb(s).
Pb2+(aq) + 2e− → Pb(s).
Q21.17C
Explain the importance of the crystal field theory and its role in the coloring of so many transition metal compounds.
S21.17C
The fact that many coordination compounds are in the form of bright colors can attributed to Crystal Field Theory. Firstly, the d-orbitals are split into two levels, one being low energy and the other being high energy, which varies for different types of structures. When a photon of visible light is absorbed, however, electrons will become excited and move from the ground state to a higher energy level (excited state). That same energy difference between the levels will therefore be directly related to the energy of the photon and inversely related to the wavelength (nm). This explains why a complex will appear to be the complementary color of its measured wavelength. Also, the strength of the ligand should be taken into consideration. Strong ligands such as CNCN will have a larger Δ, meaning absorption of a shorter wavelength. Weak ligands are quite the opposite; they tend to have smaller Δ's, which allows them to absorb a longer wavelength.
For review on this topic, visit the page "Colors of Coordination Complexes".
Q24.23C
Acetoacetic acid, CH3COCH2COOH(aq), a reagent used in organic synthesis, decomposes in acidic solution, producing acetone and carbon dioxide gas: CH3COCH2COOH(aq) → CH3COCH3(aq) + CO2(g)
This is a first-order decomposition with a half-life of 144 minutes.
How long with it take for a sample of acetoacetic acid to be 55% decomposed?
S24.23C
Start with the decomposed = 100%−55% = 45%
Next we take the natural log of our found percentage:
ln (45/100) = ln (0.45)
We know that since it's a first-order, we must use the first-order rate law ([A] = [A]o e-kt) and first-order half-life equation (t1/2 = ln(2)/ k).
Next find the value of k by rearranging the first-order half-life equation to:
k = (ln 2) * (t1/2)
= (0.693) / 144 minutes
= 0.00481 min-1
Plug the k value back in to the first-order rate law, but rearrange to solve for t
ln ([A] / [A]o) = -kt
ln (0.45) = (−0.00481 min-1) (t)
t=166 seconds
It took a sample of acetoacetic acid 166 seconds to be 55% decomposed.
Q25.10B
Fill in the blank of these nuclear reactions
- _____ + 147 N → 178O + 11H
- 19579Au + 10n → 19277Ir+ _____
- _____ + 21H → 22588Ra + 0-1β
- 21485At + _____ → 22492U + 410n
- 23588Ra + _____ → 24493Cm + 710n
S25.10B
Our goal is to fill in the blank with an element that balances the mass and atomic numbers of both sides of the arrow. We know that when two elements are added, we add the mass numbers together as well as the atomic numbers. ZAX (Z= mass number, A = atomic number).
Alpha = \(_2^4He\)
Beta (electron) = \(\text{_}_1^0\beta\)
Gamma = \(_0^0λ\)
Positron (anti-electron)= \(_1^0e\)
Neutron = \(_0^1n\)
1. On the left we have Nitrogen with a mass number of 14 and atomic number of 7. On the right side, we have Oxygen with a mass number of 17 and atomic number of 8, being added to a Hydrogen with a mass number of 1 and atomic number also of 1. On the right side, the mass numbers, 17 and 1 are added, and so are the atomic numbers of 8 and 1. After adding we get an element (Fluorine) with mass number of 18 and atomic number of 9. The blank must be added to the Nitrogen to equal the mass and atomic number of the right side. The only possible solution is Helium (He) which has a mass number of 4 and atomic number of 2.
_____ + \(_7^{14}N\) → \(_8^{17}O\) + \(_1^1H\)
\(_2^4He\) + \(_7^{14}N\) → \(_8^{17}O\) + \(_1^1H\)
2. On the left we have gold (Au) with a mass number of 195 and atomic number of 79, being added to a neutron with a mass number of 1 and atomic number of 0. On the right side, we have Iridium with a mass number of 192 and atomic number of 77. The mass numbers, 195 and 1 are added, and so are the atomic numbers of 79 and 0 for the products side. After adding we get an element (Au) with mass number of 196 and atomic number of 79. The blank must be added to Iridium to equal the mass and atomic number of the left side. The only possible solution is Helium (He) which has a mass number of 4 and atomic number of 2.
\(_{79}^{195}Au\) + \(_0^1n\) → \(_{77}^{192}Ir\)+ _____
\(_{79}^{195}Au\) + \(_0^1n\) → \(_{77}^{192}Ir\)+ \(_2^4He\)
3. On the left we have Hydrogen with a mass number of 2 and atomic number of 1. On the right side, we have Radium with a mass number of 225 and atomic number of 88, being added to a Beta (β) particle with a mass number of 0 and atomic number also of -1. On the right side, the mass numbers, 225 and 0 are added, and so are the atomic numbers of 88 and -1. After adding we get an element (Francium) with mass number of 225 and atomic number of 87. The blank must be added to the Hydrogen to equal the mass and atomic number of the right side. The only possible solution is Radon (Rn) which has a mass number of 223 and atomic number of 86.
_____ + \(_1^2H\) → \(_{88}^{225}Ra\) + \(\text{_}_1^0\beta\)
\(_{86}^{223}Rn\) + \(_1^2H\) → \(_{88}^{225}Ra\) + \(\text{_}_1^0\beta\)
4. On the left we have Astatine (At) with a mass number of 214 and atomic number of 85. On the right side, we have Uranium with a mass number of 224 and atomic number of 92, being added to four neutron (n) particle with a mass number of 1 and atomic number also of 0. On the right side, the mass numbers 224 and 4 (*Whenever there is a coefficient in front of a mass/ atomic number, you multiply each by that coefficient) are added, and so are the atomic numbers of 92 and 0. After adding we get an element (Uranium) with mass number of 228 and atomic number of 92. The blank must be added to Astatine to equal the mass and atomic number of the right side. The only possible solution is Nitrogen (N) which has a mass number of 14 and atomic number of 7.
\(_{85}^{214}At\) + _____ → \(_{92}^{224}U\) + 4\(_0^1n\)
\(_{85}^{214}At\) + \(_7^{14}N\) → \(_{92}^{224}U\) + 4\(_0^1n\)
5. On the left we have Radium (Ra) with a mass number of 235 and atomic number of 88. On the right side, we have Curium with a mass number of 244 and atomic number of 93, being added to seven neutron (n) particle with a mass number of 1 and atomic number also of 0. On the right side, the mass numbers 244 and 7 (*Whenever there is a coefficient in front of a mass/ atomic number, you multiply each by that coefficient) are added, and so are the atomic numbers of 93 and 0. After adding we get an element (Neptunium) with mass number of 251 and atomic number of 93. The blank must be added to Radium to equal the mass and atomic number of the right side. The only possible solution is Oxygen (O) which has a mass number of 16 and atomic number of 8.
\(_{88}^{235}Ra\) + _____ → \(_{93}^{244}Cm\) + 7\(_0^1n\)
\(_{88}^{235}Ra\) + \(_8^{16}O\) → \(_{93}^{244}Cm\) + 7\(_0^1n\)
S25.41D
- 9Be is the more common isotope. The other isotope of Beryllium (6Be) is radioactive, which is a good indication that it is the less stable species. Additionally, 9Be has more neutrons than protons whereas 6Be has the same number of each.
- 35Cl is, indeed, the most common isotope of Chlorine that occurs naturally. It nearly has a proton to neutron ration of 1-to-1 which is generally favored in elements with atomic numbers less than or equal to 20. More so, 43Cl is highly radioactive and decays almost immediately.
Q21.2.25
Using the information provided in Chapter 33, predict whether each reaction is favorable and the amount of energy released or required in megaelectronvolts and kilojoules per mole.
- the beta decay of bismuth-208 (mass = 207.979742 amu)
- the formation of lead-206 by alpha decay
S21.2.25
If the energy of the reactants is greater than that of the products, the reaction is spontaneous. If the energy of the products is greater than that of the reactants, the reaction is non-spontaneous. If the change in energy in Joules is positive, then the reaction is unfavorable. If the change in energy in Joules is negative, then the reaction is favorable.
1. \(_{83}^{208}Po\) → \(\text{_}_1^0\beta\) + \(_{84}^{209}Po\)
Δm = mass products - mass reactants
Δm = (207.9812457 amu + 0.000549 amu) - (207.9797422 amu)
Δm = 0.0020527 amu
(0.0020527 amu) * (931 MeV) = 1.9110637 MeV (1 amu)
(0.0020527 amu) * (1.4924 x 10-10 J) = 3.06344948 x 10-13 J (1 amu)
3.06344948 x 10-13 J * 1 kj = 3.06344948 x 10-16 kJ 1000J
This reaction is unfavorable because the change in energy in Joules is positive.
2. \(_{84}^{210}Po\) → \(_2^4He\) + \(_{82}^{206}Po\)
Δm = mass products - mass reactants
Δm = (205.9744653 amu + 4.001506 amu) - (209.9828737 amu)
Δm = -0.0069024 amu
(-0.0069024 amu) * (931 MeV) = -6.4261344 MeV (1 amu)
(-0.0069024 amu) * (1.4924 x 10-10 J) = -1.0301142 x 10-12 J (1 amu)
-1.0301142 x 10-12 J * 1 kj = -1.0301142 x 10-15 kJ 1000J
The reaction is favorable because the change in energy in Joules is negative.
Q20.4.1
Is a hydrogen electrode chemically inert? What is the major disadvantage to using a hydrogen electrode?
S20.4.1
The term inert is used to describe something that is not chemically active. Inert gases are completely inert to basic chemical reactions (ex. combustion) because their outer valence shell is completely filled with electrons. With a filled outer valence shell, an inert atom is not able to acquire or lose an electron, and is therefore not able to participate in any chemical reactions. For inert atoms or molecules, a lot of energy is involved before it can combine with other elements to form compounds.
Elemental hydrogen is inert under standard room conditions (1 atm, 1 bar, 1 M, 298 K) because the platinum does not react with the reactants (hydrogen). Being unreactive and under this condition, it only allows the inflow or outflow of electrons without reacting.
The major disadvantage of using hydrogen electrode is because it is very difficult to maintain proper concentration of H2 and H+ and the preparation of the platinum electrode.

