Oxidizing and Reducing Agents
- Page ID
- 281
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Oxidizing and reducing agents are key terms used in describing the reactants in redox reactions that transfer electrons between reactants to form products. This page discusses what defines an oxidizing or reducing agent, how to determine an oxidizing and reducing agent in a chemical reaction, and the importance of this concept in real world applications.
Oxidizing and Reducing Agents
An oxidizing agent, or oxidant, gains electrons and is reduced in a chemical reaction. Also known as the electron acceptor, the oxidizing agent is normally in one of its higher possible oxidation states because it will gain electrons and be reduced. Examples of oxidizing agents include halogens, potassium nitrate, and nitric acid.
A reducing agent, or reductant, loses electrons and is oxidized in a chemical reaction. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. A reducing agent is oxidized, because it loses electrons in the redox reaction. Examples of reducing agents include the earth metals, formic acid, and sulfite compounds.
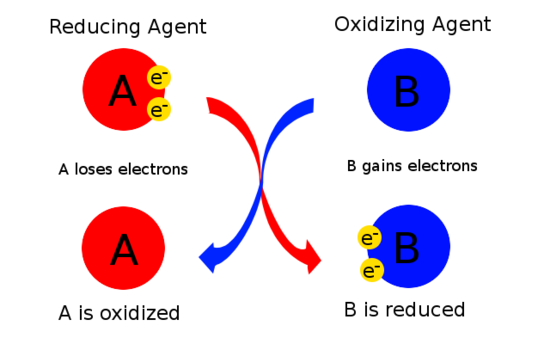
To help eliminate confusion, there is a mnemonic device to help determine oxidizing and reducing agents.
OIL RIG:
Oxidation Is Loss and Reduction Is Gain of electrons
| Common oxidizing agents | Common reducing agents |
|---|---|
| \(\ce{O2}\) | \(\ce{H2}\) |
| \(\ce{O3}\) | \(\ce{CO}\) |
| \(\ce{F2}\) | \(\ce{Fe}\) |
| \(\ce{Br2}\) | \(\ce{Zn}\) |
| \(\ce{H2SO4}\) | \(\ce{Li}\) |
| Halogens (they favor gaining an electron to obtain a noble gas configuration) | Alkali metals (they favor losing an electron to obtain a noble gas configuration) |
Identify the reducing and oxidizing agents in the balanced redox reaction:
Oxidation half reaction
Reduction Half Reaction
Overview
- Br- loses an electron; it is oxidized from Br- to Br2; thus, Br- is the reducing agent.
- Cl2 gains one electron; it is reduced from Cl2 to 2 Cl-; thus, Cl2 is the oxidizing agent.
Identify the oxidizing agent and the reducing agent in the following redox reaction:
\[\ce{MnO4^{-} + SO3^{2-} -> Mn^{+2} + SO4^{2-}}\nonumber\]
- Answer
-
\(\ce{SO3^{2-}}\) is the reducing agent and \(\ce{MnO4^{-}}\) is the oxidizing agent. Note that while a specific atom typically has an odization state changes, the agents are the actual species, not the atoms.
Applications
Oxidizing and reducing agents are important in industrial applications. They are used in processes such as purifying water, bleaching fabrics, and storing energy (such as in batteries and gasoline). Oxidizing and reducing agents are especially crucial in biological processes such as metabolism and photosynthesis. For example, organisms use electron acceptors such as NAD+ to harvest energy from redox reactions as in the hydrolysis of glucose:
\[C_6H_{12}O_6 + 2ADP + 2P + 2NAD^+ \rightarrow 2CH_3COCO_2H + 2ATP + 2NADH \nonumber\]
All combustion reactions are also examples of redox reactions. A combustion reaction occurs when a substance reacts with oxygen to create heat. One example is the combustion of octane, the principle component of gasoline:
\[2 C_8H_{18} (l) + 25 O_2 (g) \rightarrow 16 CO_2 (g) + 18 H_2O (g) \nonumber\]
Combustion reactions are a major source of energy for modern industry.
Summary
By looking at each element's oxidation state on the reactant side of a chemical equation compared with the same element's oxidation state on the product side, one can determine if the element is reduced or oxidized, and can therefore identify the oxidizing and reducing agents of a chemical reaction.
| Oxidizing Agents | Reducing Agents | |
|---|---|---|
| Oxidation State | Decreases | Increases |
| # of Electrons | Gained | Lost |
| Substance is... | Reduced | Oxidized |
Problems
- Identify the oxidizing agent and the reducing agent in the following redox reaction: \[ MnO_2(s) + 4 H^+(aq) + 2 Cl^-(aq) \rightarrow Mn^{2+} (aq) + 2 H_2O (l) + Cl_2(g)\]
- For the reaction, \(2 NO_2(g) + 7 H_2(g) \rightarrow 2 NH_3(g) + 4 H_2O(g)\), is hydrogen an oxidizing agent or a reducing agent? Explain.
- An element that is oxidized is a(n) __________ agent and an element that is reduced is a(n) __________ agent.
- Identify the reducing agent in the following redox reaction: \[ 5 SO_3^{2-} + 2 MnO_4^- + 6 H^+ \rightarrow 5 SO_4^{2-} + 2 Mn^{2+} + 3 H_2O\]
- What is the oxidizing agent in the following redox reaction? \[Zn (s) + Cu^{2+} (aq) \rightarrow Zn^{2+} (aq) + Cu(s)\]
- Determine the oxidizing and reducing agent of the following chemical equation for aerobic respiration: \[ C_6H_{12}O_6 (s) + 6 O_2 (g) \rightarrow 6 CO_2 (g) + 6 H_2O (l)\]
- For a general redox reaction involving species \(A\) and \(B\), with \(A\) losing electrons and \(B\) gaining electrons: Is A the oxidizing or reducing agent? Is B the oxidizing or reducing agent? Which one is reduced and which one is oxidized?
- In a redox reaction, there must be
- an oxidizing agent and no reducing agent
- a reducing agent and no oxidizing agent
- a reducing agent and an oxidizing agent
- no reducing or oxidizing agent
- Which of the following is a strong reducing agent? Which of the following is a strong oxidizing agent?
\(NO_3^-\), \(NO\), \(N_2H_4\), \(NH_3\)
Solutions
- \(Cl^-\) is the reducing agent because it is oxidized and loses one electron (starting with an oxidation state of -1 in the \(Cl^-\) ions and increasing to 0 in \(Cl_2\)). Remember that gaining electrons means it is "reduced". \(MnO_2\) is the oxidizing agent because it is reduced by gaining two electrons (starting with \(Mn\) in an oxidation state of +4 in \(MnO_2\) and decreasing to +2 in free \(Mn^{2+}\) ions). Keep in mind that losing electrons means it is "oxidized".
- In this reaction, hydrogen loses one electron. Hydrogen is oxidized, thus making it the reducing agent.
- An element that is oxidized is a reducing agent, because the element loses electrons, and an element that is reduced is an oxidizing agent, because the element gains electrons.
- \(SO_3^{2-}\) is the reducing agent because it loses two electrons, sulfur changes from an oxidation state of +4 in \(SO_3^{2-}\) to an oxidation state of +6 in\(SO_4^{2-}\).
- \(Cu^{2+} (aq)\) is the oxidizing agent because it gains two electrons, decreasing from an oxidation state of +2 in \(Cu^{2+} (aq)\) to an oxidation state of 0 in Cu(s).
- The oxidizing agent is oxygen and the reducing agent is glucose. Oxygen is reduced, so it is an oxidizing agent. The glucose is oxidized, so it is a reducing agent.
- When \(A\) loses electrons, it is oxidized, and is thus a reducing agent. When \(B\) gains electron, it is reduced, and is thus an oxidizing agent. \(A\) is oxidized and \(B\) is reduced.
- The answer is C: In a redox reaction, there is always an oxidizing and reducing agent
- \(NO_3^-\) is most likely to be a strong oxidizing agent. \(NH_3\) is most likely to be a strong reducing agent. This is determined by comparing the oxidation numbers of nitrogen. Because \(NO_3^-\) has the highest oxidation number of +5, compared to the other molecules, it will most likely be the oxidizing agent. Because nitrogen in \(NH_3\) has an oxidation state of -3, it has the lowest oxidation state and will most likely be the reducing agent.
References
- Gerhart, Karen. The Origins and Essentials of Life. Dubuque: Kendall/Hunt Publishing Company, 2009.
- Pettrucci, Ralph H. General Chemistry: Principles and Modern Applications. 9th. Upper Saddle River: Pearson Prentice Hall, 2007.
- Oxtoby, David W., H.P. Gillis, and Alan Campion. Principles of Modern Chemistry. 6th. Belmont: Thomson Brooks/Cole, 2008.
Contributors and Attributions
- Diana Pearson, Connie Xu, Luvleen Brar (UCD)

