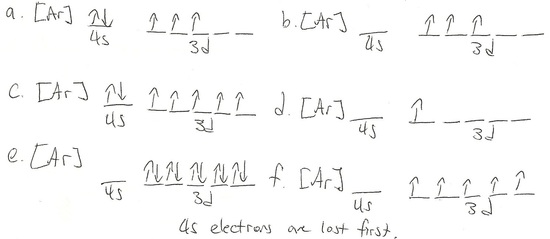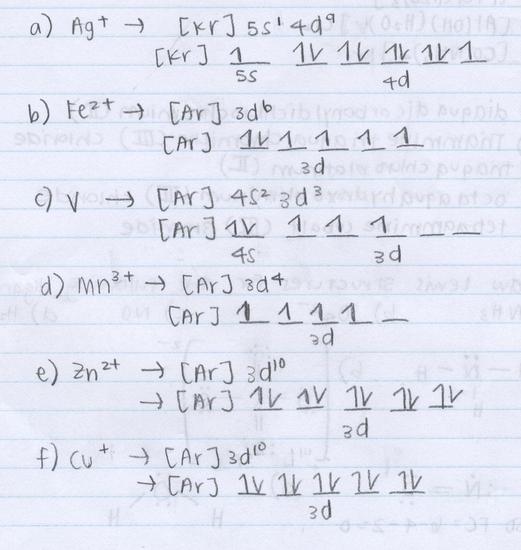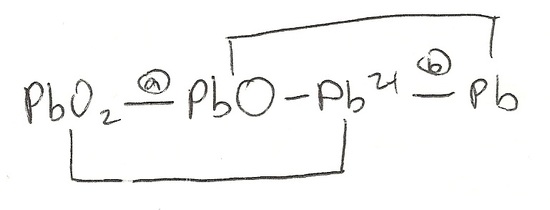20: Transition Metals
- Page ID
- 8291
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)These are homework exercises to accompany the Textmap created for "General Chemistry: Principles and Modern Applications " by Petrucci et al. Complementary General Chemistry question banks can be found for other Textmaps and can be accessed here. In addition to these publicly available questions, access to private problems bank for use in exams and homework is available to faculty only on an individual basis; please contact Delmar Larsen for an account with access permission.
Q20.1A
Draw and write out the electronic configurations of these 6 transition metal ions
- Ti4+
- V2+
- Cu
- Mn2+
- Cr3+
S20.1A
- Ti4+ [Ar]
- V2+ [Ar] 3d^3
- Cu [Ar]3d^10 4s^1
- Mn2+ [Ar]3d^5
- Cr3+ [Ar] 3d^3
Q20.1B
Write the electron configuration for the following ions: Co2+, Ni2+, Zn2+, Cu2+ and Ag+
S20.1B
- Co2+: [Ar]3d7
- Ni2+: [Ar] 3d8
- Zn2+: [Ar] 3d10
- Cu2+: [Ar] 3d9
- Ag+: [Kr]4d10
Q20.1C
Draw the orbital diagrams of the following atom and ions:
- V
- Cr3+
- Co2+
- Ti3+ (only theoretic)
- Zn2+
- Co4+ (only theoretic)
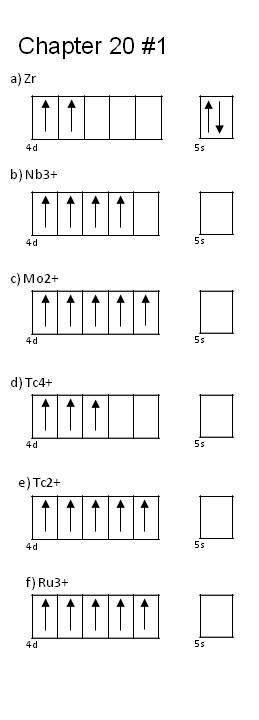
Q20.1D
By means of orbital diagrams, writing electron configurations for the following transition element atoms and ions: a) Zr; b) Nb3+; c) Mo2+; d)Tc4+; e)Tc2+; and f)Ru3+
S20.1D
- [Kr]5s2 4d2
- [Kr]4d2
- [Kr]4d3
- [Kr]4d3
- [Kr]4d5
- [Kr]4d5
To review electron configurations of transition metals, please visit "Filling Transition Metal Orbitals".
Q20.1E
Write electron configurations and orbital diagrams for the following transition element atom and ions:
- Ag+
- Fe2+
- V
- Mn3+
- Zn2+
- Cu+
S20.1E
Q20.2A
Why do transition metals often have multiple oxidation states?
S20.2A
Transition metals have both s and d electrons which are relatively easily removed. Depending on the reaction the metal loses different numbers of electrons.
Q20.3A
Describe the following trends of the periodic table
- Atomic radii
- Ionization energy
- Electronegativity
S20.3A
- atomic radii decreases across the periodic table and increases down the periodic table.
- Ionization energy increases across the periodic table and decreases down the periodic table.
- Electronegativity increases across the periodic table and decreases down the periodic table.
Q20.3B
Explain the differences between the group 1 elements and transition elements based on
- oxidation states
- colors of compounds
- magnetic properties
- formation of complexes
S20.3B
- group 1 elements all have +1 oxidation states but the transition elements can have many different oxidation states
- all alkali metals have their own flame colors but transition metals form colorful compounds due to d-d electronic transitions
- transition metals are paramagnetic when the it has unpaired electrons in the d orbital and diamagnetic when all electrons are paired. The group 1 elements are not magnetic
- group 1 elements form ionic compounds and transition elements form complexes compounds because of the many pairing possibilities in the d orbital
For review on characteristics of group 1 elements, read "Group 1: The Alkali Metals" and for review of transition elements, go to "Transition Metals and Coordination Complexes".
Q20.3C
Determine and balance the equation (the aluminum is oxidized):
\[Al_{(s)} + MoO_{3(s)} → ?\]
S20.3C
\[2Al_{(s)} + MoO_{3(s)} \rightarrow Mo_{(s)} + Al_2O_{3(s)}\]
Q20.4A
Write the half reaction for the production of Mn2+ from MnO2, a reduction half reaction in an acidic environment
S20.4A
\[MnO_{2(s)} + 4H^+_{(aq)} + 2e^- \rightarrow Mn^{2+}_{(aq)} + 2H_2O_{(l)}\]
Q20.5A
Find the electron potentials of reactions (a) and (b) of the diagram below.
S20.5A
- \(PbO_{2(s)} + 2H^+_{(aq)} + 2e^- → PbO_{(s)} + H_2O_{(l)}\)
- \(Pb^{2+}_{(aq)} + 2e^- → Pb_{(s)}\)
Q20.5B
Show the possible Lewis structure for each ligands
- CO32-
- C2O42-
- NH3
Q20.6A
Predict the molecular formulas for the following metal carbonyls:
- chromium
- ruthenium
- palladium
S20.6A
Carbonyls provide two electrons. Using the number of electrons the metal has, you can determine how many carbonyls can be used to make the total number of electrons of the metal carbonyl equal to the number of electrons as the next closest nobel gas.
- Cr(CO)6
- Ru(CO)5
- Pd(CO)4
Q20.11A
Write the balanced reaction for the thermal decomposition of \(ZnCO_{3(s)}\).
Q20.11B
What geometric structure Mer and far isomers and explain why other structure cannot have this form of isomers?
S20.11B
only octahedral can have mer and fac isomers because mer isomers nedd a 90°-90°-180° bond angle and mer isomers need 90°-90°-90°angle. This requires the geometric structure to have 6 ligands attached to it.
Q20.11C
Compete and balance the following reaction: Cr2O3(s) +Al(s) →
Cr2O3(s) +2Al(s) → Al2O3(s) +Cr(l)
S20.11C
(a) reduction: Cr2O72-(aq) + 14H+(aq) + 6e- → 2Cr3+(aq) + 7H2O(l) E०= +1.33V
oxidation: 6Fe3+(aq) + 6e- → 6Fe2+(aq) E०= 0.771V
net: Cr2O72-(aq) + 14H+(aq) + 6Fe2+(aq) → 2Cr3+(aq) + 7H2O(l) + 6Fe3+(aq)
E०cell = E०(cathode/reduction) - E०(anode/oxidation) = 1.33V - (0.771V) = 0.559V
(b) ΔG० = -nFE०cell = -(6)(96485C/mol)(0.559V) = 323610.69J/mol = 323.61kJ/mol
(c) ΔG० = -RTln(K)
323610.69J/mol = -(8.3145J/molK)(298K)ln(K)
ln(K) = -130.61
K = 1.89 x 10-57
(d) Since K is very small, this reaction will not go to completion substantially.
Please review Aluminum reaction within "Chemistry of Aluminum"
Q20.11D
Predict the following reactions and balance it:
\[Zn_{(s)}+K_2Cr_2O_{7 (s)} \rightarrow ? \]
S20.11D
'\[Zn_{(s)}+K_2Cr_2O_{7 (s)} \rightarrow ZnCr_2O_{7(s)}+ 2 K_{(s)}\]
Q20.13A
Draw mer and Fac isomers for the ZA3B3 complex. Assume A and B and different ligands and Z is a random metal
Q20.15A
Describe Chelation and how it relates to polydentates. Give an example of a polydentate.
S20.15A
Chelation is when a ligand binds to a metal more than one time. This relates to poly dentates because these ligands often chelate to metals. The example in lecture is EDTA, which Kraken due to the fact that it has binging sites 6 times to the metal.
Q20.21A
- 8H+(aq)+MnO4-(aq)+5e- → Mn2+(aq)+4H2O(l)
- 4Cr2+(aq)+O2(g)+4H+(aq) → 4Cr3+(aq)+2H2O(l)
S20.21A
half reaction: Cr2+(aq) → Cr3+(aq)+e-
Use half reactions:
MnO4-(aq) + e- → MnO42-(aq) Eo = +.56
MnO4-(aq) + 4H+(aq) + 3e- → MnO2(s) + 2H2O(l) Eo = +1.70V
to construct
MnO4- ----------MnO42-----------MnO2
↓ +.56V ↑
---------------------------------
+1.70V
Now, we just need to find the reaction from MnO42- to MnO2,
by going from MnO4- to MnO2, then from MnO2 to MnO42- we can find the route through
MnO4-(aq) + e- → MnO42-(aq) Eo = +0.56 ΔG = -nFEo = -F(0.56)V
MnO2(s) + 2H2O(l) → MnO4-(aq) + 4H+(aq) + 3e- Eocell = -1.70V ΔG = -3F(-1.70)V
then combining the equations and adding ΔG together
MnO4-(aq) + MnO2(s) + 2H2O(l) → MnO42-(aq) + MnO4-(aq) + 4H+(aq) + 2e-
cancelling out spectators
MnO2(s) + 2H2O(l) → MnO42-(aq) + 4H+(aq) + 2e- ΔG = -F(0.56 + 3(-1.70)) V
This reaction therefore has a ΔG = -F(.56 + 3(-1.70)) V which is equal to ΔG = -2FEo since it transfers two electrons, and therefore
Eo = -ΔG/(-2F) = -(-F(.56 + 3(-1.70))) / (-2F) = -(.56 + 3(-1.70))/2 = 2.27V
So, Electrode potential diagram looks like
MnO4- ----------MnO42-----------MnO2
↓ +.56V +2.27V ↑
---------------------------------
+1.70V
Q20.21B
Write half-equation to represent each of the following in an acidic solution.
- MnO4-(aq) as an oxidizing agent.
- Cr2+(aq) as a reducing agent.
Q20.21B
Write the possible half equations to represent each of the following
- Cu2+ as an oxidizing agent
- Mn2+ as a reducing agent
Q20.21C
Write an equation using \(Mn^{2+}\) ion as an oxidizing agent in basic solution. Write an equation using Fe(s) as a reducing agent.
S20.21C
- Reduction: 2e-+ MnO2(s)+2H2O(l)->Mn2+ (aq)
- Oxidation: Ag(s)->Ag+(aq)+e-
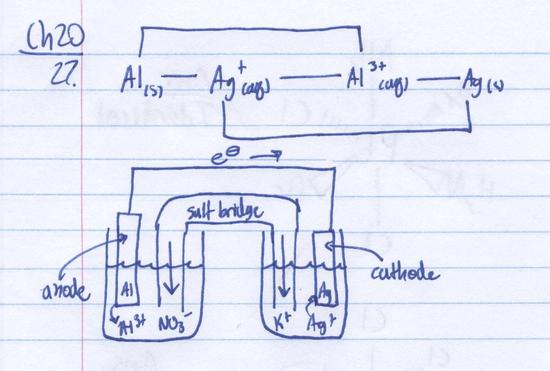
Q20.27A
Considering the following chromium species in acidic solution, assemble a standard electrode potential diagram.
Q20.27B
Create a standard electrode potential diagram for the reduction of \(Fe^{3+}\) to \(Fe^{2+}\) in acidic solution.
S20.27B
MnO4-/ MnO42- standard reduction potential is 0.56V. MnO42-/MnO2 standard reduction potential is 2.27V.
So, the overall Eo=[2(2.27V)-0.56V]/3=1.33V
Mn3+/Mn2+ standard reduction potential is 1.49V. Mn2+/Mn standard reduction potential: -1.18V
Eo=[1.49V-2(-1.18V)]/3=1.28V
Q20.57A
Predict the molecular formulas for the following metal carbonyls and explain how many electrons are contributed by the metal and how many are contributed by the carbon monoxide and which noble gas electron configuration does the molecule exhibit.
- Titanium (T)
- Manganese (Mn)
- Tungsten (W)
S20.57A
- Ti(CO)7 Ti has 22 electrons and 7 CO contribute 14 electrons for a total of 36 electrons which the same number of electron as Kr.
- Pt(CO)4; Pt has 78 electrons and 4 CO contribute 8 electrons for a total of 86 electrons which is the element Rn.
- W(CO)6; W has 74 electrons and 6 CO contribute 12 electrons for a total of 86 electrons which is the element Rn.
Q20.57B
How many electrons would be in the metals carbonyl
- Chromium
- Nickel
S20.57B
- Chromium Cr(CO)6 36
- Nickel Ni(CO)4 36
Q20.57C
What chemical formulas would you expect for the metal carbonyls of
- Chromium hexacarbonyl
- Iron Pentacarbonyl
- Nickel Tetracarbonyl
Q20.57A
What would the formulas be for the metal carbonyls of: iron, chromium, and vanadium?
- Fe(CO)5
- Cr(CO)6
- V(CO)6
To review ligand bonding, please visit the page "Ligands".