Extra Credit 18
- Page ID
- 83525
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q19.5C
Determine ΔG° for the following voltaic cell reactions:
- Pb2+(aq)+Sn(s)→Pb(s)+Sn2+(aq)
- O2(g)+2H+(aq)+2F−(aq)→H2O2(aq)+F2(g)
- Br2(l)+2Fe2+(aq)→2Br−(aq)+2Fe3+(aq)
S19.5C
To begin, ΔG° is seen as the change in Gibbs energy, or in other words, the difference in free energy between the reactants and products of a reaction. Generally we can use the equation: ΔG° = -nFE°cell where n is equivalent to the moles of electrons being transferred in the redox half reactions, F is Faraday's constant (96485C/mole-) and E°cell is the energy of the reaction found by consulting a standard reduction potential table.
To solve this question, start by separating the given equations into their respective oxidation and reduction half reactions.
a) Pb2+(aq) → Pb(s) and Sn(s) → Sn2+(aq)
Then, balance the charges of each equation with the additions of O, H and e- where needed.
Pb2+(aq) + 2e- → Pb(s)
Sn(s) → Sn2+(aq) + 2e-
Determine which reaction is the cathode and which is the anode by finding each equation's standard reduction potential on an Activity Series.
Pb2+(aq) + 2e- → Pb(s) ⇒ -0.126V Anode
Sn(s) → Sn2+(aq) + 2e- ⇒ 0.14V Cathode
E°cell can then be found using: E°cell = E°cathode - E°anode.
E°cell = (0.14) - (-0.126) = 0.266V
Using this information we an put together the final equation for ΔG°.
ΔG° = -nFE°cell
ΔG° = (-2mole-)(96485C/mole-)(0.266V) = -51330.02J
b) O2(g) + 2H+(aq) + 2F-(aq) → H2O2(aq) + F2(g)
Separate and balance each equation with the additions of O, H and e- where needed.
O2(g) + 2H+(aq) + 2e- → H2O2(aq)
2F-(aq) → F2(g) + 2e-
Determine which reaction is the cathode and which is the anode by finding each equation's standard reduction potential on an Activity Series.
O2(g) + 2H+(aq) + 2e- → H2O2(aq) ⇒ 0.68V Cathode
2F-(aq) → F2(g) + 2e- ⇒ -2.87V Anode
E°cell can then be found using: E°cell = E°cathode - E°anode.
E°cell = (0.68) - (-2.87) = 3.55V
Using this information we an put together the final equation for ΔG°.
ΔG° = -nFE°cell
ΔG° = (-2mole-)(96485C/mole-)(3.55V) = -685043.5J
c) Br2(l)+2Fe2+(aq)→2Br−(aq)+2Fe3+(aq)
Separate and balance each equation with the additions of O, H and e- where needed.
Br2(l) + 2e- → 2Br-(aq)
2Fe2+(aq) → 2Fe3+(aq) + e-
Determine which reaction is the cathode and which is the anode by finding each equation's standard reduction potential on an Activity Series.
Br2(l) + 2e- → 2Br-(aq) ⇒ 1.06V Cathode
2Fe2+(aq) → 2Fe3+(aq) + e- ⇒ -0.77V Anode
E°cell can then be found using: E°cell = E°cathode - E°anode.
E°cell = (1.06) - (-0.77) = 0.8162V
Using this information we an put together the final equation for ΔG°.
ΔG° = -nFE°cell
ΔG° = (-2mole-)(96485C/mole-)(0.8165) = -157502.114J
For more help on this topic, refer to: Libretexts: Electrochemistry and Thermodynamics.
Q19.45C
The following voltaic cell is constructed:
Pb(s) || Pb2+ (satd PbI2) || Pb2+ (0.100M) | Pb(s). Given that the Ecell = 0.0567V, find the Ksp.
S19.45C (not sure if I did this right)
To solve this problem, begin by setting up the Nernst equation using the appropriate values. E°cell in this case would be 0, two moles of electrons are transferred.
Pb(s) → Pb2+(aq) + 2e-
Thus, the Nernst equation sets up as 0.0567 = E°cell - (0.0592 / n)x(logQ)
Q = (x/0.100)
Plug in the values of E°cell, n and Q, and solve for the value of x.
0.0567 = 0 - (0.0592 / 2)x(log(x/0.100))
1.916 = log(x/0.100)
-e1.916 = (x/0.100)
x = -0.6791
This value tells you the amount of Pb2+ that needs to be saturated. To determine Ksp, use the values of [Pb] and [I−]2. Use the Pb and I concentrations to solve this value.
Ksp = [Pb][I−]2 and Ksp = 1.79×10−9.
For further help with his topic, refer to: Libretexts: Nernst Equation.
Q21.3C
Name these complex compounds:
- [CoCl(H2O)2(NH3)3]I2
- [CrBr2(CO)2(NO)2]+
- K2[Fe(CN)4]
- [CuI2BrCO]2−
- [Fe(ox)Cl2(H2O)]−
S21.3C
- triamminediaquachlorocobalt(III) iodide
- dibromodicarbonyldinitrosylchromium(III) ion
- potassium tetracyanoferrate(II)
- bromocarbonyldiiodocuprate(I) ion
- aquadichlorooxalatoferrate(III) ion
For further review of this topic, refer to: Libretexts: Nomenclature of Coordination Complexes.
Q24.10A
At 65°, the half-life for the first-order decomposition of N2O5(g) is 2.35 minutes.
N2O5(g)→2NO2(g)+12O2(g)
If 1.00g of N2O5 is introduced into an evacuated 10L flask at 65°C,
- What is the initial partial pressure, in mmHg, of N2O5(g)?
- What is the initial partial pressure, in mmHg, of N2O5(g) after 2.35 minutes?
- What is the total gas pressure, in mmHg, after 2.35 minutes?
S24.10A (not sure if this is right)
1. To solve for the initial partial pressure of N2O5(g), start by using the ideal gas law equation: pV = nRT. Where R is the constant: 0.08206 (L*atm/mol*K)
pV = nRT becomes p = nRT/V
n = number of moles of N2O5 which can be found using the molar mass of the compound (108.1g) and the amount introduced to the flask (1.00g).
n = 1.00/108.1 = 0.00925mols
T is measured in Kelvin meaning that we have to add 273.15 to the given temperature in °C.
T = 65 + 273.15 = 338.15K
⇒ p = (0.00925)(0.08206)(338.15)/(10)
p = 0.02567atm
Then convert to the appropriate units (mmHg)
p - 0.02567atm = (0.02567)(760) = 19.5087mmHg
2. Given that the half life of the compound is 2.35 minutes, we can use the half life equation to find the partial pressure at this time:
2.35 = ln(2)/k
k = 1.629
ln(x)/19.5 = (-1.629)(2.35) = -0.693
x = -0.534mmHg
3. Then use these values to solve for the total pressure, given that one half life has passed.
x / 19.5 = 0.5
x = 9.75mmHg
For more help on this topic, refer to: Libretexts: Rate Laws.
Q24.49A
Given the following graph:
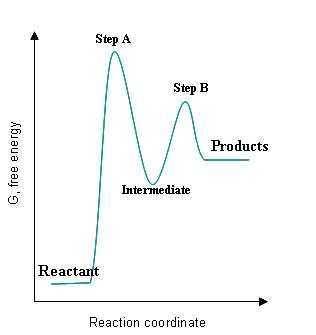
Please answer the following,
- How can you tell where the intermediate is on the graph?
- How can you tell where the transition state(s) is/are on the graph?
- How can you tell where the fastest step of the reaction is on the graph?
- How can you tell which step has the smallest rate constant?
- How can you tell whether the steps of the reaction are exothermic or endothermic?
- How can you tell whether the entire reaction is exothermic and endothermic?
S24.49A
- On the graph, the intermediate is a local minimum that is brought to the transition state before being converted to the final products.
- Step A and Step B are local maximums that signify the activation energy that must be reached for reactants to proceed through the reaction before forming final products.
- The step with the smallest activation energy will be the fastest step of the reaction, which would be Step B since the energy gap between Step B and the intermediate is significantly smaller than the gap between Step A's transition state and the reactant.
- The slowest reaction with the largest activation energy would have the smallest rate constant, which would be Step A.
- If the reactants have a higher energy than the products, it can be predicted that energy was released and the reaction is exothermic; if the reactants have a lower amount of energy than the products, the reactants gained heat and the reaction is endothermic.
- The entire reaction can be seen as exothermic if the energy of the final products is less than the energy of the reactants; likewise, if the energy of the final products is greater than that of the initial reactants, the reaction can be seen as endothermic.
For further information regarding this topic, refer to: Libretexts: Reaction Coordinate Diagrams.
Q25.25E
Suppose that a sample of Mg23 (Half-life = 11.32s) has an activity 500 times the detectable limit. How long could experiments be run before radiation falls below detectable limits?
S25.25E
To solve this problem, we need to find the time (t) at which the sample of Mg23 surpasses the detectable limit of radiation (1/500 of the starting value).
We start by finding the rate constant.
λ = 0.693/11.32s = 0.0612
Relate the rate of decay to time and sample amount using the equation:
ln(1) - ln(500) = -(0.0612s-1)(t)
Solve for t:
t=101.514s
Q18.5
For each of the following reactions: 1) find E° (in volts) and 2) determine whether the reaction is spontaneous under standard conditions.
- the reaction between iron and iron(III) ions to give iron(II) ions.
- the following cell: I−|I2||Zn2+|Zn
S18.5
First, recall that E°cell = E°Cathode - E°Anode.
Begin by writing out the equation for each of the reactions.
Identify which is the cathode (reduction) and anode (oxidation).
1. Fe3+ + e- → Fe2+
2. 2I- → I2 + 2e-
Zn2+ + 2e- → Zn
Use a standard reduction potential table to determine the E° for each half reaction and use them to determine the overall E°cell for each reaction.
1. E°cell = 0.771
2. E°cell = (-.535) - (-0.763) = 0.228
Knowing that when E°cell > 0 the reaction is spontaneous and when E°cell < 0 the reaction is non-spontaneous allows us to determine:
1. Spontaneous reaction
2. Spontaneous reaction
For more information regarding electrochemistry, visit: Libretexts: Electrochemistry Basics.
Q21.4.2
If a sample of one isotope undergoes more disintegrations per second than the same number of atoms of another isotope, how do their half-lives compare?
S21.4.2
Assuming the half life equation of a first order reaction, we can estimate that t1/2 = ln(2)/k. Now, if we were to say that the one isotope, isotope A, undergoes a certain amount of disintegrations per second and isotope B undergoes more disintegrations per second than isotope A, then we can quantify:
ka < kb ; k being the rate of disintegrations per second.
Since ka < kb, t1/2 of isotope A would be larger than that of isotope B.
Thus, the more disintegrations per second an isotope has, the shorter its half life will be.

