21.S: Carboxylic Acid Derivatives (Summary)
- Page ID
- 207032
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Concepts & Vocabulary
21.0 Chapter Objectives and Introduction to Carboxylic Acid Derivatives
- Carboxylic acid derivatives formed when the hydroxyl group of the carboxylic acid is replaced by a different group.
- Carboxylic acid derivatives are also known as acyl derivatives.
- Many types of carboxylic acid derivatives, the chapter focused on acid halides (acyl halides), anhydrides, esters, amides, thioesters, and acylphosphates.
- Cyclic esters are referred to as lactones and cyclic amides are referred to as lactams.
- Nucleophilic acyl substitution reactions convert the carboxylic acid to one of the derivatives.
21.1 Naming Carboxylic Acid Derivatives
- Acid halide are named following the IUPAC rules with the carboxylic acid ending with -oyl or -yl and the halide ending in an -ide.
- Anhydrides are named following the IUPAC rules with the carboxylic acid ending -acid being replaced by -anhydride.
- Esters are named following the IUPAC rules with the carboxylic acid ending with -oic acid being replaced by -ate and the alkoxy alkyl chain as a substituent.
- Thioesters are named following the IUPAC rules with the carboxylic acid ending with -oic acid being replaced by -thioate and the sulfide alkyl chain as a substituent.
- Primary amides are named following the IUPAC rules with the carboxylic acid ending with -oic acid being replaced by -amide.
- Secondary or tertiary amides are named as primary amides are, but in additon an upper case N is used to designate the alkyl groups attached to the nitrogen atom, which are names as substituents.
- Cyclic amides (lactams) use a Greek letter to identify the location of the nitrogen in relation the carbonyl group.
- Acyl phosphates are named following the IUPAC rules with the carboxylic acid ending with -oyl or -yl and -phosphate.
21.2 Nucleophilic Acyl Substitution Reactions
- Carboxylic acid derivatives contain an electronegative heteroatom (typically oxygen, nitrogen, sulfur, or phosphorus) bonded directly to the carbonyl carbon represented by the symbol Y.
- The acyl group is the remainder of the molecule, which includes the carbonyl and the attached alkyl group.
- The Y group acts as a leaving group during the nucleophilic acyl substitution reaction.
- Stability of the negative charge on the Y group can be guaged by the pKa of its corresponding acid (HY).
- Carboxylic acid derivatives undergo a reaction called nucleophilic acyl substitution.
- The electrophile is the carbon of the carbonyl, which undergoes an attack by a nucelophile followed by the elimination of the Y group in the carboxylic acid derivative.
- The Y group is substituted for the nucleophile.
- The reactivity of the carboxylic acid derivatives depends on the stability of the carbonyl and the effectiveness of the leaving group.
- Stabilization of the carbonyl reduces the electrophilic character of the carbonyl group.
- The ability of the leaving group to stabilize negative charge creates a more effective leaving group.
- More reactive carboxylic acid derivatives can be used to make less reactive carboxylic acid derivatives.
21.3 Nucleophilic Acyl Substitution Reactions of Carboxylic Acids
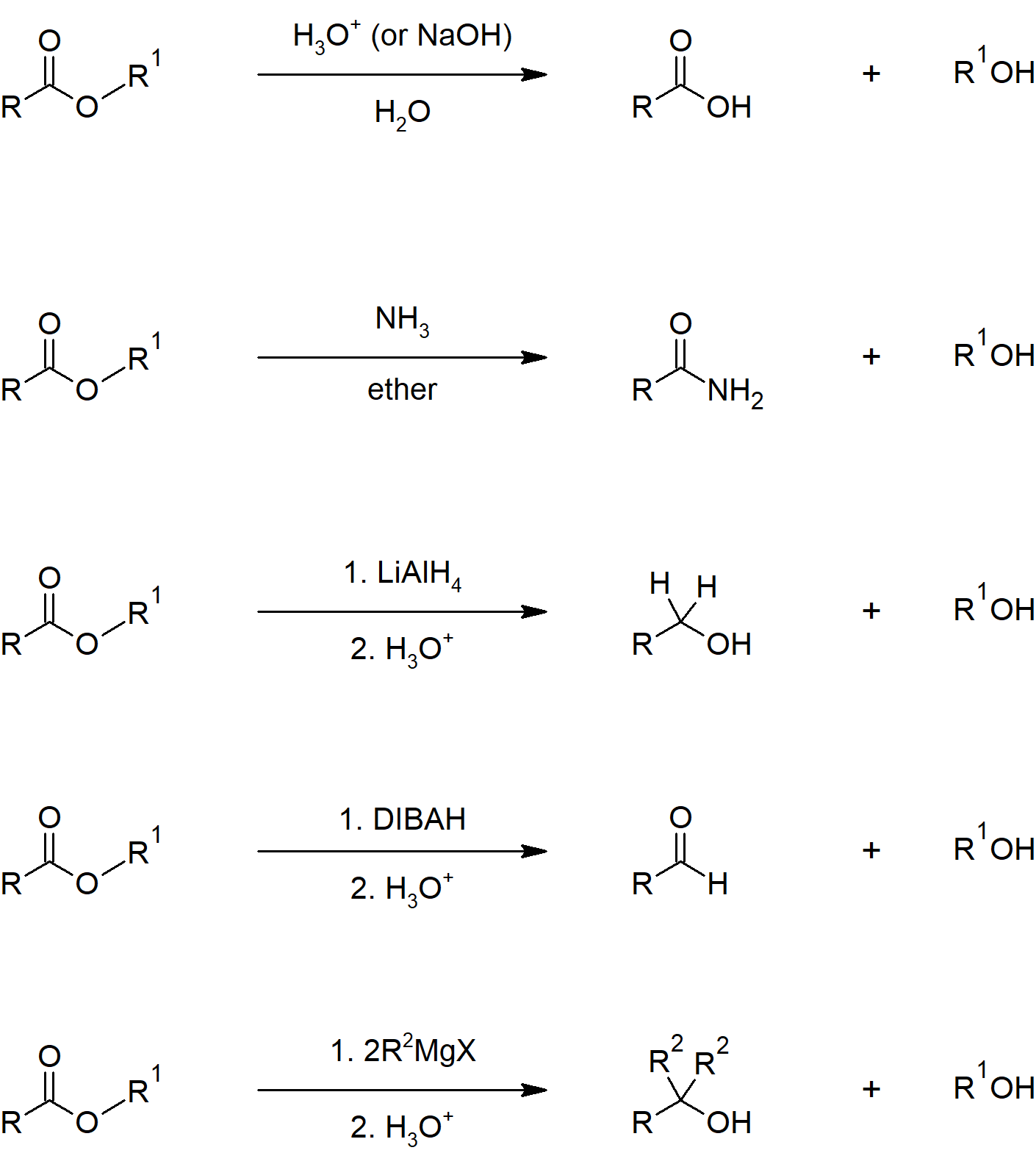
- The hydroxyl of a carboxylic acid is a poor leaving group, but activating the carbonyl and converting the hydroxyl to a better leaving group allow for reactions to occur.
- Carboxylic acids can be converted to acid chlorides using thionyl chloride (SOCl2).
- Carboxylic acids can be converted into anhydrides by condensing two carboxylic acids together and the loss of water.
- Carboxylic acids can be converted into esters in two ways.
- Using the carboxylate in an SN2 mechanism.
- The Fischer Esterification
- Acid catalyzed
- Pushing equilibrium in the forward direction using Le Chatelier's principle
- Carboxylic acids can be converted into amides in two ways.
- Direct conversion by heating ammonium carboxylate salts
- Using a coupling agent like dicyclohexylcarbodiimide (DCC)
- Carboxylic acids can be reduced to primary alcohols using lithium aluminum hydride or borane tetrahydrofurane (THF).
- In biological chemistry, carboxylate anions are converted to an acyl phosphate, which requires phosphrylation using TAP as the phosphate donor or thioesters using coenzyme A.
21.4 Chemistry of Acid Halides
- Carboxylic acids react with thionyl chloride to form acid chlorides.
- Carboxylic acids react with phosphorus tribromide to form acid bromides.
- Acid chlorides are the most reactive carboxylic acid derivative, which allows them to easily convert to other acyl compounds.
- Acid chlorides undergo hydrolysis to form carboxylic acids.
- Acid chlorides react with carboxylic acid to from anhydrides through a nucleophilic acyl substitution.
- Acid chlorides react with alcohol nucleophiles to produce esters with pyridine being used to neutralize the HCl produced.
- Acid chlorides can be reduced to aldehydes using hindered reducing agents that have only one equivalent of hydride.
- Acid chlorides react with amines to form amides, usually an excess of amine is used to neutralize the HCl produced.
- Acid chlorides react with Grignard reagents to from tertiary alcohols.
- Acid chlorides react with Gillman reagents to produce ketones.
21.5 Chemistry of Acid Anhydrides
- Anhydrides generally made by nucleophlic acyl substitution reactions using an acid chloride and a carboxylic acid or carboxylate anion.
- Anhydrides are not as reactive as acid chlorides, but still undergo many of the same reactions.
- Asymmetrical anhydrides not used to make amides or esters since multiple products can be formed.
- Anhydrides undergo hydrolysis to form carboxylic acids.
- Anhydrides react with alcohol nucleophiles to produce esters.
- Anhydrides can react with amines to form the corresponding amides.
- Esters are present in many biologically important molecules.
- Esters are often the sources of pleasant aromas of many fruits and perfumes.
- Esters are also present in commercial and synthetic applications.
- Esters are prepared
- most often using nucleophilic acyl substitution using an acid chloride and an alcohol
- using nucleophilic acyl substitution using an anhydride and an alcohol
- using nucleophilic acyl substitution using a carboxylic acid and an alcohol
- deprotonating a carboxlylic acid for an SN2 reaction
- Esters can be converted to a carboxylic acid through a reaction witt water and a catalytic amount of strong acid.
- Saponification is the reaction between an ester and water under basic conditions to produce a carboxylate anion.
- Esters can be inter-converted using a transesterfication reaction.
- Esters can be converted into amides using an ester and a large excess of amine.
- Esters can be reduced to primary alcohols using lithium aluminum hydride.
- Like acid chlorides, esters can be reduced to aldehydes using weak, bulky reducing agents.
- Esters can react with Grignard reagents to form tertiary alcohols.
- Amides make up the backbone of proteins when the amino group of one amino acid reacts with the carboxylate carbon of another amino acid and from an amide linkage (peptide bond).
- Amides are most commonly prepared through the reaction of an acid chloride and amine.
- Amides are relatively unreactive towards nucleophilic acyl substitutions due to the poor leaving group ability of its nitrogen containing Y group.
- Amides can be hydrolzyed to a carboxylic acid and ammonia or amine by heating the reaction under acidic or basic aqueous solutions.
- Hydrolysis of amines is the first step in metabolism of dietary proteins.
- Primary amines can be converted into a nitrile through a reaction with thionyl chloride.
- Amides are reduced to amines by treatment with lithium aluminum hydride.
21.8 Chemistry of Thioesters and Acyl Phosphates - Biological Carboxylic Acid Derivatives
- Acyl phosphates, acyl adenosine phosphophates, and thioesters are all activated forms of carboxylate groups in biochemical reactions.
- Carboxylate groups of fatty acids are converted to thioesters using coenzyme A
- Thioesters are a good substrate for a number of metabolic transformations.
- Thioesters can be hydrolyzed, resulting back to a carboxylate anion.
- Thioesters and acyl phosphates are the most reactive biologically relevant acyl groups, but not as reactive as an acid chloride or anhydride.
21.9 Polyamides and Polyesters - Step-Growth Polymers
- Step-growth is one of two broad classes of polymerization methods.
- Step-growth polymerizations are linked by a carbon-heteroatom bond formed in non-sequential steps.
- A step-growth polymerization starts with two complementary functional groups on different monomers reacting to form a dimer.
- Using a difunctional monomer, allows step-growth polymers to grow in two directions at once.
- Fibers are made from some form of polymer.
- Nylon
- First successful fully synthetic polymer was nylon-6,6 in 1938 by the company DuPont, which is a polyamide.
- Nylons are widely used synthetic fibers, which are found in commercial products such as rope, sails, clothing, and more.
- Esterfication
- Can also be used to form primary linkages in step-growth polymers.
- Polyester is typically produced when a dicarboxylic acid and a diol react together.
- The most important polyester is polyethylene terephthalate (PET), which is used to make bottles for soda.
- Polyesters are fibers often used in many fabrics.
- Polycarbonates
- Monomer is a carbonate, which acts like a double ester and can undergo a type of double transesterification reaction with two alcohols.
- A carbonate is difunctional and can react with a diol to form polymers called polycarbonates.
- Used in engineering because they are strong materials, but easily molded.
- Polycarbonates are found in DVDs, compact discs, and Blu-ray discs.
21.10 Spectroscopy of Carboxylic Acid Derivatives
- IR of acid chlorides shows a strong carbonyl stretch around 1800 cm-1.
- IR of anhydrides show two strong carbonyl stretch around 1750 and 1820 cm-1.
- IR of esters shows a strong carbonyl stretch around 1740 cm-1 and a strong CO-O stretch around 1150 to 1250 cm-1.
- IR of amides shows a strong carbonyl stretch around 1700 cm-1, primary and secondary amides have two bands and tertiary amides have one band and a strong N-H stretch around 3170 to 3500 cm-1 in primary and secondary amides.
- 1H NMR of acid halides do not have any distinctive peaks.
- 1H NMR of anhydrides do not have any distinctive peaks, but symmetrical anhydrides will produce equivalent protons on either side.
- 1H NMR of esters and thioesters have an alkyl group attached to the heteroatom. For esters, the alkoxide protons will show up in the 3.5-4.5 ppm region, while those attached to the sulfur in thioesters will show up in the 2.0-3.0 ppm region.
- 1H NMR of amides have the possibility of alkyl groups or hydrogens attached. Resonances for protons attached to the carbon bonded to the nitrogen of the amide resonate between 2.0-3.0 ppm region. The NH protons of primary and secondary amides resonate in the 7.5-8.5 ppm regions, which will be broad.
- 13C NMR of carboxylic acid derivatives show the carbonyl carbon at 160-180 ppm.
- 13C NMR of esters also have the carbon attached to the alkoxide of the ester, which appears at 50-90 ppm.
- 13C NMR of amides also have the carbon attached to the nitrogen of the amide, which appears at 20-65 ppm.
- 13C NMR of thioesters also have the carbon attached to the sulfur, which appears at 20-45 ppm.
- Mass spectra of carboxylic acid derivatives show a base peak due to the acylium ion.
Skills to Master
- Skill 21.1 Name carboxyilic acid derivatives using IUPAC rules.
- Skill 21.2 Draw the structure of carboxylic acid derivatives from the IUPAC name.
- Skill 21.3 Compare the stability of different carboxylic acid derivatives to determine reactivity.
- Skill 21.4 Determine the products for nucleophilic acyl substitution reactions.
- Skill 21.5 Provide step-by-step mechanism for the Fischer esterification.
- Skill 21.6 Show how to synthesize an ester from a carboxylic acid and alcohol.
- Skill 21.7 Show mechanisms for the different carboxylic acid derivatives starting from an acid chloride.
- Skill 21.8 Show how to synthesize the different carboxylic acid derivatives starting from an acid chloride.
- Skill 21.9 Provide step-by-step mechanisms for the different carboxylic acid derivatives starting from an anhydride.
- Skill 21.10 Show how to synthesize the different carboxylic acid derivatives starting from an anhydride.
- Skill 21.11 Understand why the hydrolysis of an ester is not reversible under basic conditions and why the reverse does not produce an ester.
- Skill 21.12 Be able to synthesize tertiary alcohols from esters.
- Skill 21.13 Provide a step-by-step mechanism for the hydrolysis of an amide under acidic and basic conditions.
- Skill 21.14 Provide the structure of different polymers using the bracketed notation and the monomers from the polymer.
- Skill 21.15 Use IR, NMR, and MS to identify different carboxylic acid derivatives.
Summary of Reactions
Carboxylic Acid Reactions
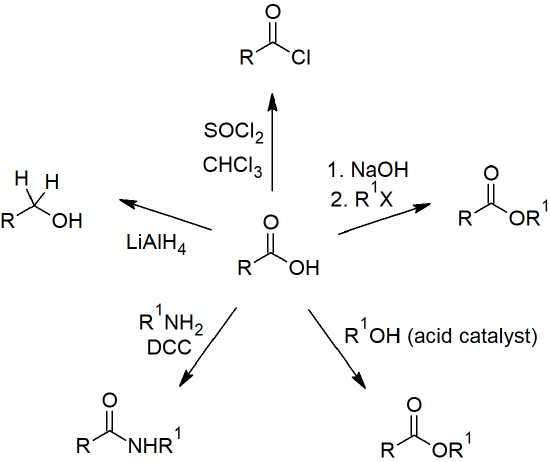
Amide Reactions
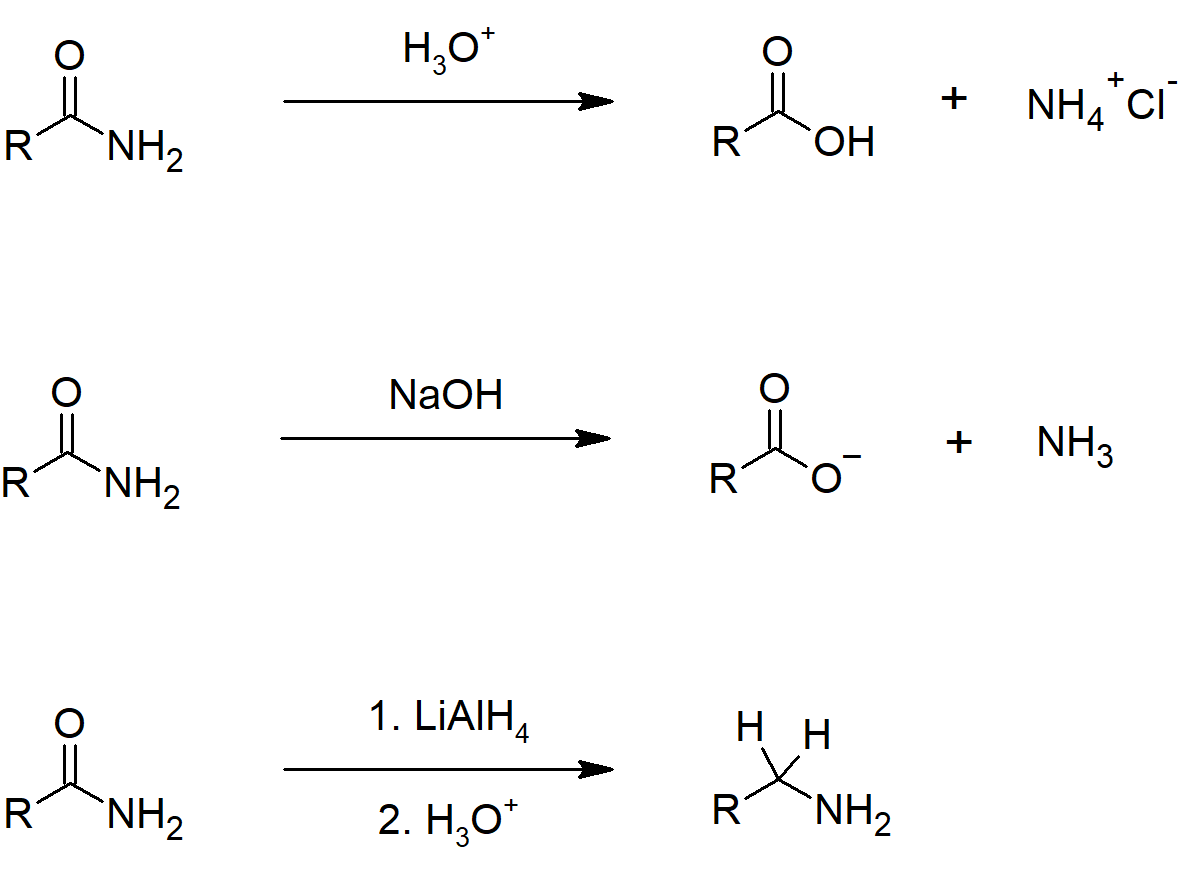
Acid Chloride Reactions
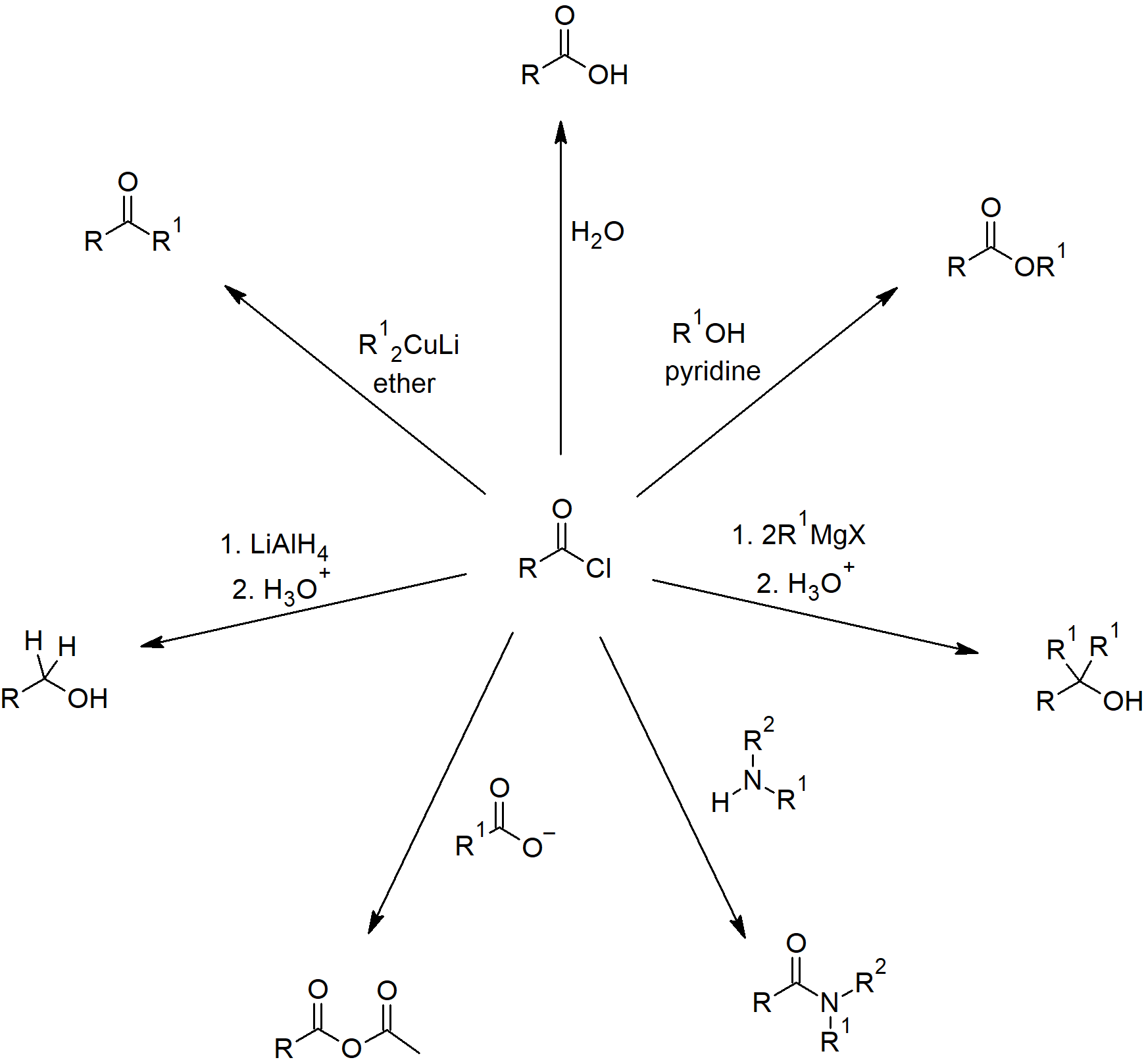
Acid Anhydride Reactions
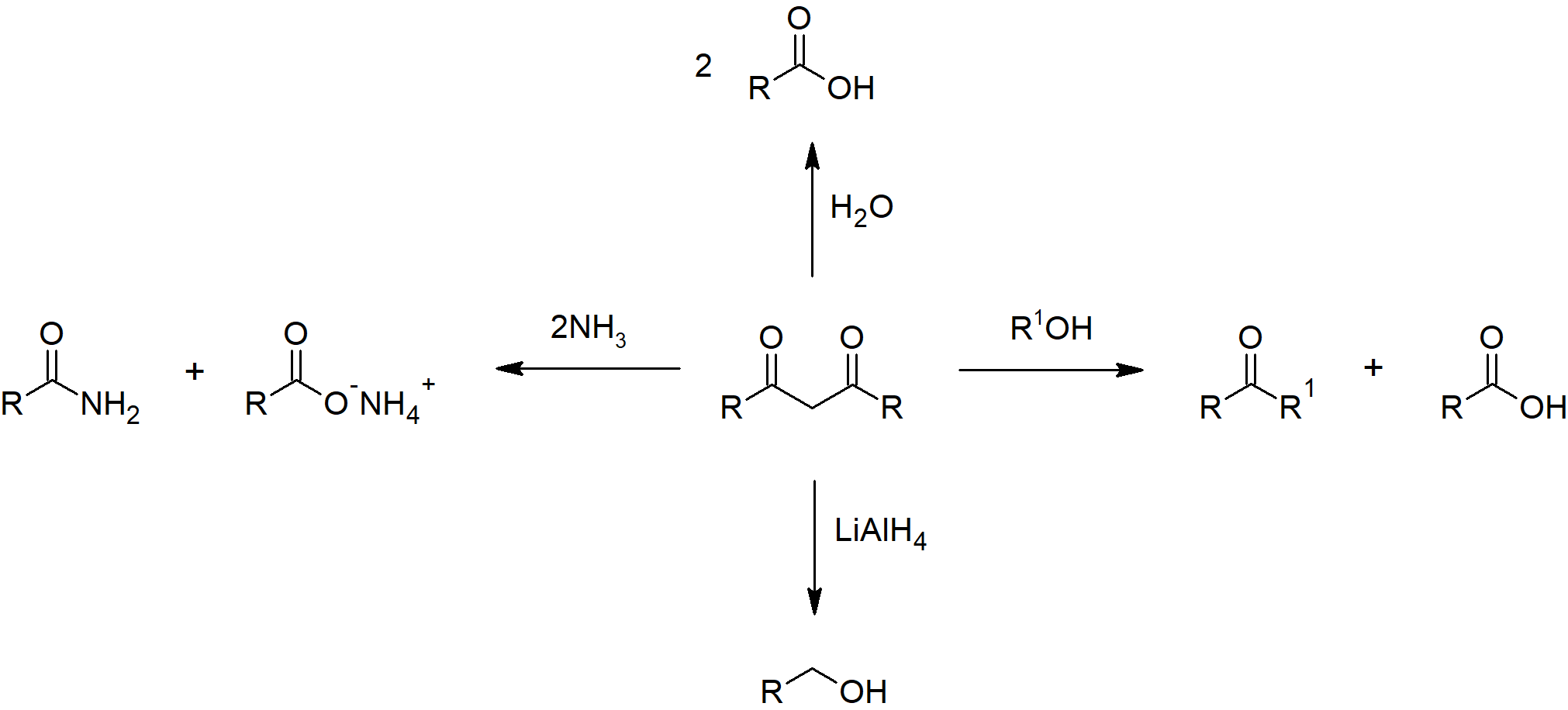
Ester Reactions