21.5: Chemistry of Acid Anhydrides
- Page ID
- 36402
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- write an equation to illustrate the preparation of an acid anhydride from an acid halide and the sodium salt of a carboxylic acid.
- identify the product formed from the reaction of a given acid halide with the sodium salt of a given carboxylic acid.
- identify the acid halide, carboxylate salt, or both, required to prepare a given acid anhydride.
- write an equation to describe the reaction of an acid anhydride with each of the following: water, alcohol, ammonia, a primary or secondary amine, lithium aluminum hydride.
- identify the product formed when a given acid anhydride is reacted with any of the reagents listed in Objective 4, above.
- write a detailed mechanism for the reaction of an acid anhydride with any of the reagents listed in Objective 4, above.
- identify the acid anhydride, the nucleophilic reagent, or both, needed to prepare a specified carboxylic acid, ester, amide, or primary alcohol.
The reactions described in this section are, in principle, identical to those discussed in Section 21.4. Once you have understood the mechanism of nucleophilic acyl substitution, these reactions should not present you with any great difficulty, and memorization can be kept to a minimum.
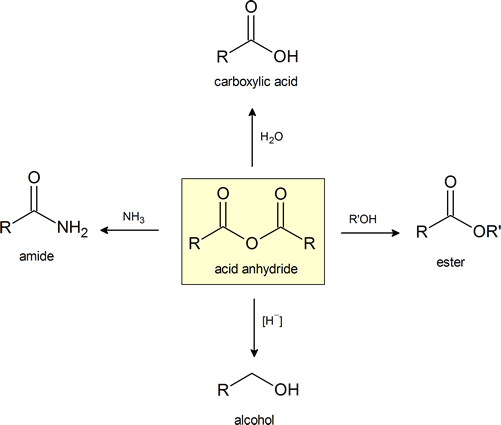
This figure provides a convenient general summary of a few of the reactions described in Section 21.5. Note that from a synthetic perspective the ester‑ and amide‑forming reactions are the most common, so they are the focus of this section.
Preparation of Acid Anhydrides
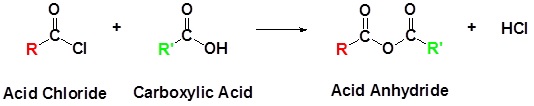
As show in Section 21.4, acid anhydrides are generally made using a nucleophilic acyl substitution reaction of an acid chloride with a carboxylic acid or a carboxylate anion.
Chemistry of Anhydrides
Anhydrides are highly reactive to nucleophilic attack and undergo many of the same reactions as acid chlorides that were explored in section 21.4. Although slower reacting than acid chlorides, anhydrides react with water to form carboxylic acids, with alcohols to form esters, and with amines to form amides. Anhydrides can also be reduced to 1o alcohols by hydride reduction. Because many anhydrides are made from the coupling of two carboxylic acids, reactions using anyhydrides waste one equivalent of the carboxylic acid as a leaving group. As a consequence, reactions are only commonly performed with inexpensive, readily available anhydrides, such as acetic anhydride or benzoic anhydride. Anydrides have the advantage of being easier to work with than acid chlorides.

Generic Reaction with an Anhydride
Generic Mechanism of Nucleophilic Acyl Substitution using an Anhydride
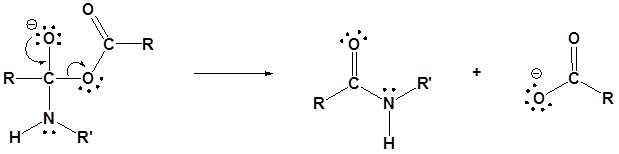
The following nucleophilic acyl substitution reactions are all similar and can be represented by one generic mechanism. The nuceophile (water, ammonia, amine, or alcohol) adds to one of the carbonyl carbons in the anhydride forming a tetrahedral alkoxide intermediate. The reforming of the carbonyl C=O bond eliminates a carboxylate leaving group. Because the nucleophile is neutral, a protonated intermediate is formed. A base deprotonates the intermediate to form the neutral carboxylic acid derivative product (carboxylic acid, ester, amide).
1) Nucleophilic attack
2) Deprotonation
3) Leaving group removal
Predicting the Products of an Anhydride Reaction
An anhydride and a neutral nucleophile react to form a new acyl compound through nucleophilic acyl substitution. During this reaction there are three changes in bonding. The leaving group is removed from the anhydride. The neutral nucleophile loses a hydrogen. A C-Nuc bond is formed between the nucleophile and the anhydride’s electrophilic carbonyl carbon.
Reactions with Asymmetrical Anhydrides
Asymmetrical anhydrides are typically not used in the formation of esters and amides because they have the possibility of forming two different products. This is not the case when symmetrical anhydrides are used.
Conversion of Acid Anhydrides to Carboxylic Acids: Hydrolyisis
Anhydrides react rapidly with water to form two carboxylic acids compounds. Because anhydrides are often prepared from carboxylic acids this reaction serves little synthetic value. However, this reaction serves as a reminder to prevent the exposure of anhydrides to moisture because they will become contaminated with the corresponding carboxylic acids.
General Reaction
Example
Conversion of Acid Anhydrides to Esters: Alcoholyisis
Anhydrides react with alcohols to form esters as the main product and a carboxylate as a side product. The reaction is typically run with a base, such as NaOH or pyridine, to remove any acid produced. Notice that one acyl group from the anhydride is incorporated into the ester and the other acyl group forms a carboxylate.
General Reaction
Example
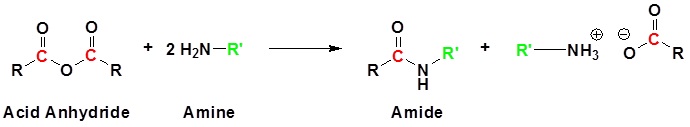
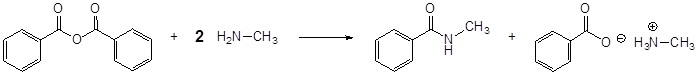
Conversion of Acid Anhydrides to Amides: Aminolysis
Acid anhydrides react with ammonia, 1o, or 2o amines to form the corresponding amides. Two molar equivalents of amine are required. It is important to run this reaction with a base to neutralize the acid produced otherwise the amine reactant would be become protonated to form a non-nucleophilic ammonium compound. During the reaction, one acyl group from the anhydride becomes part of the amide while the other acyl group becomes a carboxylate.
Draw the products of the following reaction:
Solution
The reaction of an anhydride and an amine form an amide through nucleophilic acyl substitution. During this reaction three changes in bonding occur. The leaving group is removed from the anhydride. The amine loses a hydrogen. A C-N bond is formed between the amine and the electrophilic carbonyl carbon.
Acid Anhydrides react with alcohols to form esters
Reactions of anhydrides use Pyridine as a solvent
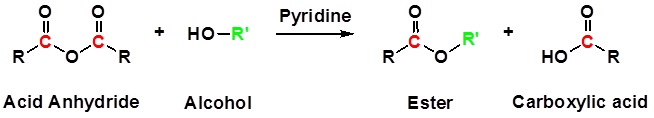

Mechanism
1) Nucleophilic Attack by the Alcohol
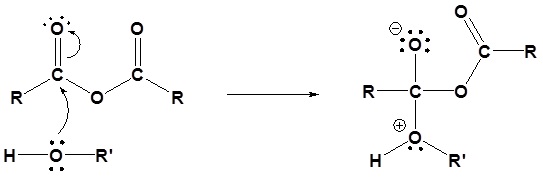
2) Deprotonation by pyridine
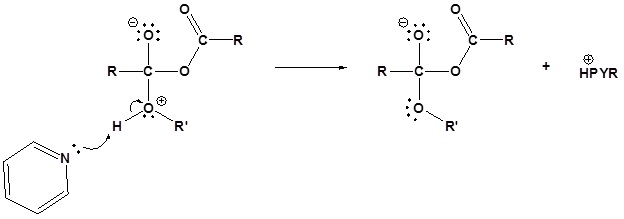
3) Leaving group removal
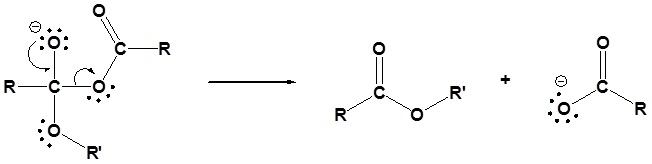
4) Protonation of the carboxylate
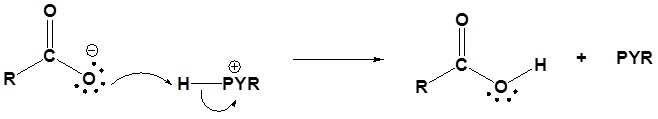
Acid Anhydrides react with amines to form amides
Mechanism
1) Nucleophilic Attack by the Amine
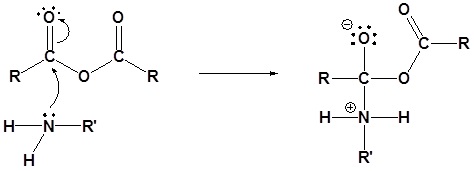
2) Deprotonation by the amine
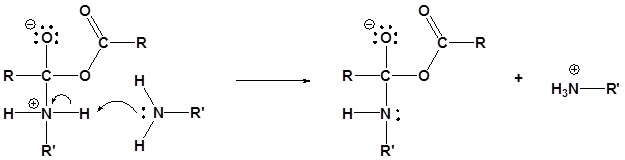
3) Leaving group removal