21.1: Naming Carboxylic Acid Derivatives
- Page ID
- 36398
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- provide a satisfactory name for an acid halide of given structure.
- draw the condensed or shorthand structure of an acid halide, given either its commonly used or systematic name.
- provide a satisfactory name for an acid anhydride of given structure.
- draw the condensed or shorthand structure of an acid anhydride, given either its trivial or IUPAC name.
- provide an acceptable name for an amide of given structure.
- draw the condensed or shorthand structure of an amide, given its trivial or IUPAC name.
- provide an acceptable name for an ester of given structure.
- draw the condensed or shorthand structure of an ester, given either its trivial or IUPAC name.
- provide an acceptable name for a thioester of given structure.
- draw the condensed or shorthand structure of a thioester, given its trivial or IUPAC name.
- provide an acceptable name for an acyl phosphate.
- draw the condensed or shorthand structure of an acyl phosphate, given either its trivial or IUPAC name.
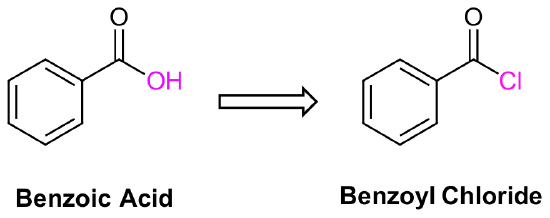
Nomenclature of acid halides, RCOX
The nomenclature of acid halides starts with the name of the corresponding carboxylic acid. If the corresponding carboxylic acid has an –oic acid or –ic acid ending it is removed and replaced with the ending -oyl followed by the first syllable of the name of the halogen along with an –ide ending.
However there are a number of specific exceptions where IUPAC allows the ending -yl to be used instead. Examples are formic ⇒ formyl, acetic ⇒acetyl, propionic ⇒propionyl, butyric ⇒butyryl, oxalic ⇒ oxalyl, and succinic ⇒ succinyl.
When the corresponding acid includes a -carboxylic acid ending, it is removed and replaced with the ending -carbonyl. This is followed by the first syllable of the name of the halogen along with an –ide ending
The carbonyl carbon is given the #1 location number. The acid halide functional group is assumed to be on the end of the parent chain, so it is not necessary to include the functional group location number in the name.

Nomenclature of Anhydrides, RCO2COR'
An anhydride functional group results when two carboxylic acids combine and lose water (anhydride = without water). The names of anhydrides come from the names of the two combined carboxylic acids. The carbonyl carbon is given the #1 location number on both chains. The anhydride functional group is assumed to be on the end of the each parent chain, so it is not necessary to include the functional group location number in the name.
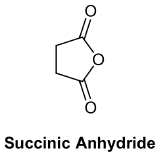
Symmetrical anhydrides made from unsubstituted carboxylic acids and cyclic anhydrides made from dicarboxylic acids are named based on their corresponding carboxylic acid. The ending -acid is replaced with -anhydride. This is true for both the IUPAC and common nomenclature. An anhydride is symmetrical when it is acyclic and the acyl R groups are the same.
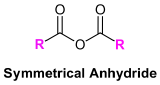
Example
An unsymmetrical anhydride is created from two different carboxylic acids. For unsymmetrical anhydrides, name both acids alphabetically with a space between them. Then add the end word anhydride.
Nomenclature of Esters, RCO2R'
Esters consist of two distinctly different carbon chains which need to be named separately. One carbon chain is from the corresponding carboxylic acid and the other chain, attached to the singly bonded oxygen, is called the alkoxy alkyl chain.
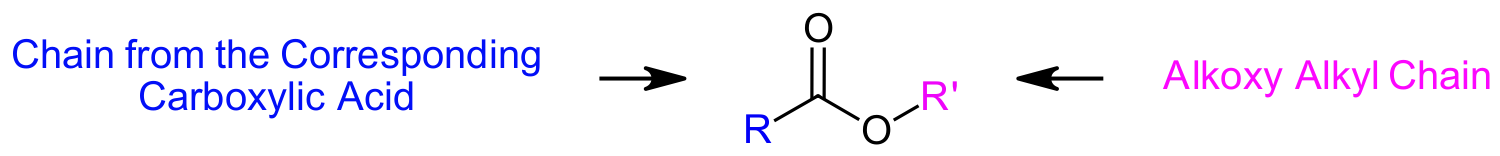
Esters are named as if the alkoxy alkyl chain is a substituent (Prefix + yl). This is followed by the name of the corresponding carboxylic acid part of the ester with -ic acid or -oic acid replaced with the ending –ate. The carbonyl carbon is given the #1 location number. The carbonyl functional group is assumed to be on the end of the parent chain, so it is not necessary to include the functional group location number in the name.
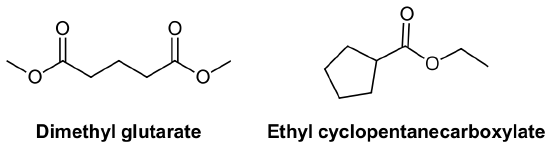
Nomenclature of Thioesters, RCOSR'
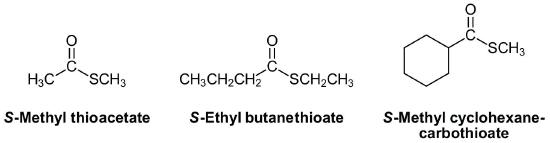
Thioesters have two distinctly different carbon chains. One carbon chain is from the corresponding carboxylic acid and the other chain, attached to the sulfur, is called the sulfide alkyl chain.
Thioesters are named as if the sulfide alkyl chain is a substituent with the letter S preceding (S-Prefix + yl). This is followed by the parent chain of the corresponding carboxylic acid, named as an alkane with the ending –thioate added. For thioesters attached to a carbon ring the ending -carboxylic acid is replaced with -carbothioate. When using the common names of the carboxylic acid the -ic acid ending is replaced -ate and the prefix thio- is added.
The carbonyl carbon is given the #1 location number. The carbonyl functional group is assumed to be on the end of the parent chain, so it is not necessary to include the functional group location number in the name.
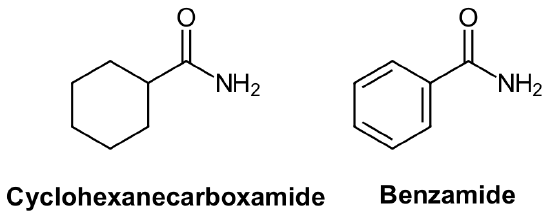
Nomenclature of Amides, RCONH2, RCONHR', RCONR'R''
Primary amides (RCONH2) are named by changing the name of the corresponding acid by removing the -oic acid or -ic acid endings and adding -amide. Amides derived from a cyclic carboxylic acid have the -carboxylic acid ending replaced with -carboxamide. The carbonyl carbon is given the #1 location number. It is not necessary to include the location number in the name because it is assumed that the functional group will be on the end of the parent chain.
Example
Secondary (RCONHR') and tertiary (RCONR'R'') amides are named by using an upper case N to designate that the alkyl groups are attached to the nitrogen atom. These alkyl groups are named as substituents (Prefix + yl).
Cyclic amides are called lactams. A Greek letter identifies the location of the nitrogen on the alkyl chain relative to the carboxyl carbonyl group.
Nomenlcature of Acyl Phosphates, RCOOPO32-
Acyl phosphates are named following the rules as acid halides except name of the halogen is replaced with the word phosphate. If there is an alkyl group attached to one of the phosphate oxygens, it is named as a substituent (parent + yl) and listed before the end word phosphate.
When a phosphate ion is attached to a carbon atom on an organic molecule, the chemical linkage is referred to as a phosphate ester, and the whole species is called an organic monophosphate. Glucose-6-phosphate is an example.
If an organic molecule is linked to two or three phosphate groups, the resulting species are called organic diphosphates and organic triphosphates.
Isopententyl diphosphate and adenosine triphosphate (ATP) are good examples:
Oxygen atoms in phosphate groups are referred to either 'bridging' and 'non-bridging', depending on their position. An organic diphosphate has two bridging and five non-bridging oxygens.
When a single phosphate is linked to two organic groups, the term 'phosphate diester' is used. The backbone of DNA is composed of phosphate diesters.
The term 'phosphoryl group' is a general way to refer to all of the phosphate-based groups mentioned above.
Phosphate groups on organic structures are sometimes abbreviated simply as 'P', a convention that we will use throughout this text. For example, glucose-6-phosphate and isopentenyl diphosphate are often depicted as shown below. Notice that the 'P' abbreviation includes the oxygen atoms and negative charges associated with the phosphate groups.
| Functional Group | Structure | Suffix Name |
|---|---|---|
| Carboxylic acid | -oic acid | |
| Carboxylate | -oate | |
| Ester | -oate | |
| Dicarboxylic acid | -dioic acid | |
| Acyl halide | -oyl halide | |
| Anhydride | -ic anhydride | |
| Amide | -an amide | |
| Acyl Phosphate | -oyl phosphate | |
| Nitrile | -ane nitrile |
Exercises
1) Name the following compounds using IUPAC conventions
(a)
(b)
(c)
(d)
(e)
(f)
(g)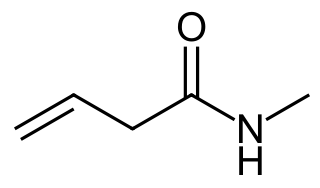
(h)
(i)
2) Draw structures corresponding to the following names:
- benzoic anhydride
- phenyl hexanoate
- methyl benzoate
- 3-chloro-N-ethylbenzamide
- pentanamide
- N-phenylethanamide
- Methyl 1-methylcyclohexanecarboxylate
- Ethyl 3-oxopentanoate
- S-Methyl p-bromothiobenzoate
- Formic propanoic anhydride
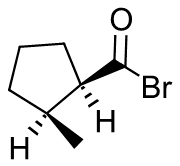
- cis-2-Methylcyclopentanecarbonyl bromide
Solutions
1)
- 2-methylpentanoyl chloride
- 2-
cyclopentylacetamide - propyl 2-methylpropanoate
- cyclohexylbutanoate
- sec-butyl cyclopentanecarboxylate
- 1-methylbutylcyclopentane carboxylate
- N-methyl-3-butenamide
- (S)-2-hydroxypropanoyl phosphate
- S-propyl 2,3-dimethyl-2-butenethioate
2)
a. 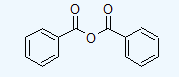
b. 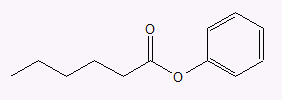
c. 
d. 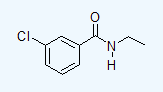
e. 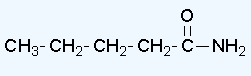
f.
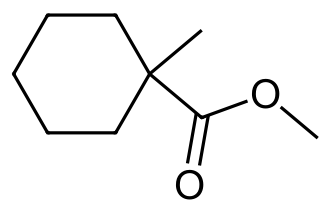
g.
h. 
i. 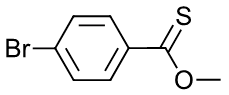
j. 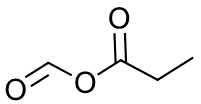
k.