1.2: What is a chemical bond
- Page ID
- 394085
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe the nature of ionic, covalent, and coordinate covalent bonds.
- Understand the nature of a bond's polarity and molecules' polarity.
- Describe the nature of intermolecular forces and their effect on some properties of the compounds.
- Compare the properties of organic and inorganic compounds.
Chemical bonds were introduced in general chemistry. This is a review of chemical bonds for understanding the differences between inorganic and organic compounds. The chemical bonds connect the atoms in the compounds. Transferring or sharing some valance electrons from one atom to another makes the bonds. There are three major types of chemical bonds, ionic, covalent, and coordinate covalent bonds, as described below.
Ionic bond
An ionic bond is formed by transferring some valence electrons from one atom (usually from a metal atom) to another (usually to a nonmetal atom). The atom that loses an electron becomes a positively charged specie called a cation. For example, sodium atom \(\ce{Na}\) loses one electron and become \(\ce{Na^{+}}\), or calcium \(\ce{Ca}\) loses two electrons and becomes \(\ce{Ca^{2+}}\). The atom that gains an electron becomes a negatively charged specie called an anion. For example, chlorine atom \(\ce{Cl}\) gains one electron and become \(\ce{Cl^{-}}\), or oxygen \(\ce{O}\) gains two electrons and becomes \(\ce{O^{2-}}\).
The same charges repel each other, and opposite charges attract each other. The cations and the anions come together in the structure of the compound in such a way that the distances between the centers of opposite charges are minimized to increase attraction. The spaces between the centers of the same charges are maximized to decrease repulsion, as illustrated in Figure \(\PageIndex{1}\) for the case of \(\ce{NaCl}\). An ionic bond is the net attractive force holding the ions together in an ionic compound. ionic compounds are hard and have high melting and boiling points due to the strong electrostatic attractive forces. Ionic compounds are brittle because a slight displacement of one layer of atoms relative to the other layer can place similar charges next to each other and split due to repulsive forces between similar charges.

Covalent bond
What is a covalent bond?
A bond formed by sharing valence electrons is a covalent bond. Each bonded atom contributes one electron in a shared pair of electrons called a covalent bond, e.g., \(\ce{H-Cl}\) bond illustrated in Figure \(\PageIndex{2}\). The shared pair of electrons is called a bonding pair, and it is usually represented as a single line between the bonded atoms as in \(\ce{H-Cl}\). Note that there are three unshared pairs of electrons on \(\ce{Cl}\) atom in \(\ce{H-Cl}\) molecule as illustrated in Figure \(\PageIndex{2}\). The unshared electron pairs are called nonbonding or lone pairs of electrons. Atoms may share two electrons each to make a double bond, e.g., in \(\ce{O=O}\), or three electrons each to create a triple bond, e.g., in \(\ce{N≡N}\), as illustrated in Figure \(\PageIndex{2}\).

How is a covalent bond formed?
Isolated atoms have potential energy due to several factors, like movements of atoms, movements of and electrostatic interactions among subatomic particles, etc. When two atoms approach each other, attractive and repulsive forces develop. The attractive forces are the attraction between opposite charges, i.e., between the nucleus of one atom and the electrons of the other. The repulsive forces are the repulsion between the same charges. When the two atoms can place more electrons between the nuclei by overlapping their atomic orbitals, the attractive forces between nuclei and electrons become stronger than the repulsive forces. Figure \(\PageIndex{3}\) illustrates this situation for the case of \(\ce{H-H}\) covalent bond formation. The two atoms move towards each other due to the attractive force resulting in a decrease in their potential energy until the minimum potential energy is reached. Decreasing the internuclear distance to less than the bond distance results in repulsive forces increasing more than attractive forces, and the overall potential energy increases.
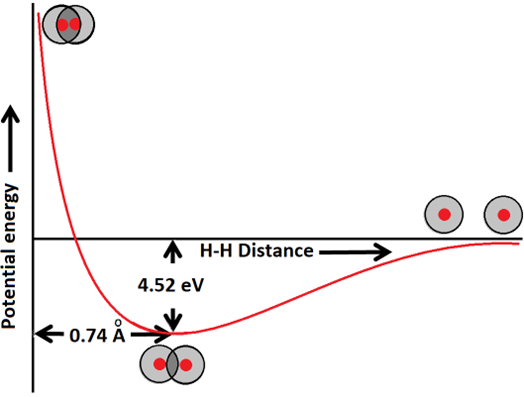
- Bond lenght is the distance between the centers of the two atoms at the potential energy minimum. For example, the bond distance \(\ce{H-H}\) is 0.74 Å.
- The difference in the energy of isolated atoms and the energy state at the bonding distance is the bond energy. For example, the bond energy of \(\ce{H-H}\) is 4.52 eV as shown in Figure \(\PageIndex{3}\)
- Bond energy is always released when the bond is formed, and the same amount of energy is absorbed when the same bond is broken. Stronger bonds have higher bond energy, and weaker bonds have lower bond energy.
The polarity of a covalent bond
The polarity was introduced in general chemistry. Here is a quick review. When the bonded atoms are the same, the bonding electron pair equally shared between them and the bond is nonpolar. For example, \(\ce{H-H}\), \(\ce{F-F}\), and \(\ce{C-C}\) bonds are nonpolar. When the bonded atoms differ, one atom attracts the bonding election more than the other. The ability of an atom to attract the bonding electrons to itself is called electronegativity. If the difference in the electronegativity values of the bonded atoms is less than 0.5, the bond is still considered nonpolar. However, if the difference in the electronegativity values of the bonded atoms is between 0.5 to 1.9, the bond is a polar covalent. When the electronegativity difference is more than 1.9, it is usually an ionic bond.
In a polar covalent bond, the more electronegative atom has a partial negative (\(\delta\)-) charge because the electronegative atom has more share of negatively charged electrons, and the other atom has partial positive (\(\delta\)+) charge, e.g., \(\ce{\overset{\delta{+}}{H}{-}\overset{\delta{-}}{Cl}}\), \(\ce{\overset{\delta{+}}{C}{-}\overset{\delta{-}}{O}}\), and \(\ce{\overset{\delta{-}}{O}{-}\overset{\delta{+}}{H}}\).
The polarity of a molecule
The polarity of molecules is described in general chemistry. Recall that polarity is a vector quantity. It is also shown as an arrow over the bond with the arrowhead pointing to \(\delta\)- end and the tail with a plus sign starting from \(\delta\)+ end of the polar bond, e.g., \(\ce{\overset{\delta{+}}{H}{-}\overset{\delta{-}}{Cl}}\) can be represented in vector form as: \(\ce{\overset{\Large{+\!->}}{H-Cl}}\). Suppose there is more than one polar bond in a molecule. In that case, the polarity vectors may cancel out each other, e.g., two equal and opposite polarity vectors in a carbon dioxide molecule (\(\ce{\overset{\large{<-\!++\!->}}{O=C=O}}\)) cancel each other resulting in a nonpolar molecule. Therefore, the molecule with more than one polar bond may or may not be polar, depending on whether the polarities cancel each other. The following rules determine the polarity of a molecule:
- A molecule is polar if there is only one polar bond in it.
- A molecule is polar if there are polar bonds, but the molecule is not symmetric. In this case, the polarities do not entirely cancel out.
- A molecule is nonpolar if there are polar bonds, but the molecule is symmetric, e.g., i) linear with two equal bonds, ii) trigonal planar with three equal bonds, iii) tetrahedral with four equal bonds. In these cases, the polarities cancel out.
- A molecule is nonpolar if there is no polar bond in it.
Coordinate covalent bond
The coordinate covalent bond is the same as the covalent bond, except that both bonding electrons are donated by one of the two bonded atoms. The coordinate covalent bond is also called a dative bond. For example, the bond formed between the boron atom of boron trifluoride (\(\ce{BF3}\)) and the nitrogen atom of ammonia (\(\ce{ _{\bullet}^{\bullet}\! NH3}\)) in the following reaction: \(\ce{F3B + _{\bullet}^{\bullet}\! NH3 -> F3 \overset{-}{B}- \overset{+}{N}H3}\), where \(\ce{N}\) donates its lone pair to make the bond. The charges developed on the \(\ce{N}\) and \(\ce{B}\) after bonding is explained later in the formal charge section.
Intermolecular interactions
Although a covalent bond is almost as strong as an ionic bond, the covalent bond forces operate within a molecule. The question is, what holds the molecules together in solid molecular compounds? These are electrostatic interactions called intermolecular forces that were introduced in general chemistry. Intermolecular forces are much weaker than ionic, covalent, or coordinate covalent bonds. A line shows a covalent bond, while dotted lines usually show intermolecular forces. Three major intermolecular forces exist: i) London dispersion forces, ii) dipole-dipole interaction, and iii) hydrogen bonding.
London dispersion
Atoms have +ve protons in the nucleus and equal -ve electrons outside the nucleus. The atom is nonpolar because the electrons and protons are equal, and the -ve electrons are symmetrically distributed around the +ve nucleus. Electrons can be considered like clouds that can temporarily sway to one side or the other. If the electron cloud sways to one side, the atom is no more nonpolar, the side of the nucleus becomes a partial positive (\(\delta\)+) pole, and the side where electrons move to becomes a partial negative (\(\delta\)-) pole. This transient dipole in one atom induces a temporary dipole in the neighboring atom due to repulsion between the same charges or attraction between the opposite charges. The dipoles orient themselves to maximize attractive force between opposite charges and minimize repulsion between the same changes resulting in a net attractive force. This attraction between transient dipole-induced dipole is called the London dispersion force. The same phenomenon happens in the molecules.
The transient dipole appears and disappears randomly. London dispersion force is proportional to molecular mass because more mass means more electrons and a higher probability of temporary dipole. That is why smaller nonpolar organic compounds, like methane (\(\ce{CH4}\), boiling point ~-160 oC) are gases, large like decane (\(\ce{C10H22}\), boiling point ~+174 oC) are liquid, and even larger like eicosane (\(\ce{C20H42}\), melting point ~37 oC, boiling point ~+343 oC) are solid.
Dipole-dipole interactions
Polar molecules have a permanent dipole in addition to the London dispersions force (transient dipoles). Therefore, polar molecules have higher intermolecular interaction and, consequently, higher melting and boiling points than nonpolar molecules of comparable molecular masses. For example, acetone (\(\ce{C2H6O}\)), a polar molecule, is liquid (melting point ~-95 oC and boiling point ~+56 oC), and butane (\(\ce{C4H10}\)), a nonpolar molecule of the same molecular mass (58 g/mole), is a gas (melting point ~-137 oC and boiling point ~0 oC).
Hydrogen bonding
Hydrogen bonding is a particular class of dipole-dipole interactions that involve \(\ce{\overset{\delta{-}}{O}{-}\overset{\delta{+}}{H}}\), \(\ce{\overset{\delta{-}}{N}{-}\overset{\delta{+}}{H}}\), or \(\ce{\overset{\delta{-}}{F}{-}\overset{\delta{+}}{H}}\) dipole. Hydrogen bonding is usually stronger than dipole-dipole interactions because i) the dipoles in hydrogen bonding are usually stronger than the other dipoles, ii) \(\delta{+}\) charge is denser on a small \(\ce{H}\) than the same charge on larger atoms, and iii) being small, \(\ce{H}\) can penetrate even in tight spaces to establish hydrogen bonding. For example, methanol (\(\ce{CH3OH}\)), capable of hydrogen bonding, is a liquid (boiling point ~+65 oC), formaldehyde (\(\ce{H2C=O}\)), a polar molecule without hydrogen bonding capability, is a gas (boiling point ~-19 oC), and ethane (\(\ce{C2H2}\)), a nonpolar molecule, is a gas with much lower boiling point (~-89 oC), all three having comparable molar masses 32 g/mole, 30 g/mole, and 30 g/mole, respectively. Further, hydrogen bonding is more important in living things because \(\ce{H}\) is one of the primary constituents of organic compounds.
The intermolecular forces play an essential role in the chemistry of living things, e.g., as illustrated in Figure \(\PageIndex{4}\) for defining the shape of a protein molecule.

Comparison of bonding and properties of organic and inorganic compounds
Organic compounds
- Organic compounds are usually covalently bonded molecules, e.g., methane (\(\ce{CH4}\)), glucose (\(\ce{C6H12O6}\)), etc.
- Organic compound are primarily composed of \(\ce{C}\) and \(\ce{H}\). Other elements, like \(\ce{O}\), \(\ce{N}\), \(\ce{P}\), \(\ce{S}\), etc., may also be present in small amounts.
- The polarity of organic compounds varies from nonpolar to highly polar, e.g., decane (\(\ce{C10H22}\)) is nonpolar, and glucose (\(\ce{C6H12O6}\)) is polar.
- Organic compounds are usually insoluble in water, e.g., decane (\(\ce{C10H22}\)) is insoluble in water, except a few highly-polar compounds that are soluble, e.g., glucose (\(\ce{C6H12O6}\)) is soluble in water.
- Organic compounds usually have lower melting and boiling points, e.g., decane (\(\ce{C10H22}\)) melts at ~-30 oC and boils at ~174 oC.
- Organic compounds are usually soft solids, liquids, or sometimes gases, e.g., glucose (\(\ce{C6H12O6}\)) is a soft solid, decane (\(\ce{C10H22}\)) is liquid, and methane (\(\ce{CH4}\)) is a gas.
Inorganic compounds
- Inorganic compounds are usually ionic or highly polar covalent, e.g., table salt (\(\ce{NaCl}\)) is ionic and ammonium phosphate (\(\ce{(NH4)3PO4}\) -a fertilizer, is a combination of covalent and ionic.
- Inorganic compounds are usually composed of a combination of metals and nonmetals. e.g., calcium carbonate (\(\ce{CaCO3}\) is composed of metal (\(\ce{Ca}\)) and nonmetals (\(\ce{C}\)) and (\(\ce{O}\)).
- Inorganic compounds are usually soluble in water, e.g., \(\ce{NaCl}\) and \(\ce{(NH4)3PO4}\) are soluble in water. Some exceptions exist, e.g., \(\ce{CaCO3}\) is not soluble in water.
- Inorganic compounds usually have high melting and boiling points, e.g., \(\ce{NaCl}\) melts at ~-801 oC and boils at ~1465 oC.
- Inorganic compounds are usually hard and brittle solids, with few liquids and gases, e.g., \(\ce{NaCl}\) and \(\ce{CaCO3}\) are hard and brittle solids.


