3.1: Bonding in compounds
- Page ID
- 372888
Chemical bonds
Compounds are a pure form of matter formed by atoms of more than one element combined in a constant whole number ratio.
The bonds connect the atoms in the compounds. Sharing or transferring some valance electrons from one atom to the other makes the bonds. Noble gases have a full valence shell of eight valence electrons, except helium which has a full valence shell of two valence electrons. The noble gases are the least reactive, i.e., the most inert group of elements.
The octet rule states that atoms of all elements other than noble gases tend to share, lose, or gain valence electrons to acquire the electron configuration of the nearest noble gas having eight valence electrons.
Covalent bonds
A bond formed by sharing electrons is a covalent bond.
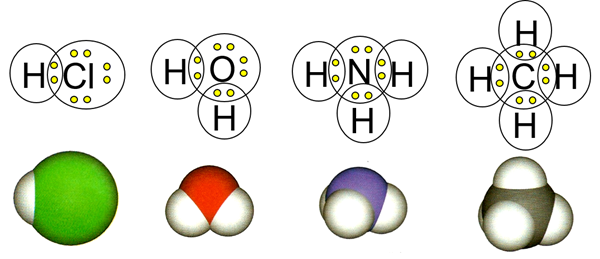
When a nonmetal atom combines with another nonmetal atom, they usually make a covalent bond. A covalent bond is a pair of shared electrons, called a bonding pair of electrons, where each bonded atom contributes one electron. For example, chlorine has seven valence electrons and needs one more to complete its octet. Hydrogen has one valence electron and requires one more to acquire the electron configuration of helium, i.e., duet instead of the octet. Hydrogen and chlorine combine by sharing one electron to make the compound HCl. Similarly, oxygen, nitrogen, and carbon make 2, 3, and 4 covalent bonds with hydrogen to complete their octet and make compounds H2O, NH3, and CH4, respectively, as illustrated in Fig. 3.1.1.
Two atoms can share one, two, or three electrons to make a single, a double, or a triple covalent bond. For example, H2 has a single bond (H-H), O2 has a double bond (O=O), and N2 has a triple bond (N≡N), where each line between the atoms represent one covalent bond. Fig. 3.1.2 illustrates the formation of three covalent bonds in N2. A valence electron pair that is not involved in bonding is called a nonbonding pair. One nonbonding pair and three bonding pairs complete the octet of each nitrogen atom in the N2 molecule, as shown in Fig. 3.1.2.
The bonding pair of electrons counts towards the total valence electrons of each bonded atom., i.e., in H-H each hydrogen atom has two valence electrons, and in :N≡N: each nitrogen has eight valence electrons; two in the nonbonding pair and six in three bonding pairs.

The formula of a covalent compound
A compound is represented by a chemical formula that combines the symbols of its constituent elements. More electropositive elements are usually written first, e.g., HF, NO, CO. Some exceptions to this rule, e.g., NH3 and CH4, have more electronegative elements written first. The majority of the covalent compounds exist as discrete molecules. A subscript to the right of the element’s symbol represents the number of atoms of the component of the molecule. For example, H2O has two hydrogen atoms and one oxygen atom in a water molecule. Note that subscript 1 is not written, i.e., the symbol of an element alone represents one atom.
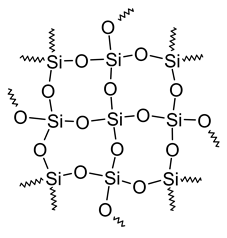
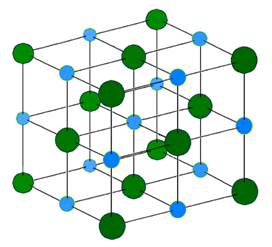
A few covalently bonded compounds are giant molecules where the atoms are held together by a 3D network of bonds. The formula of these compounds shows the simplest whole-number ratio of elements in the compound. For example, Fig. 3.1.3 shows SiO2 present in high-purity sand and quartz, a giant molecule.

Ionic bond
A bond formed by the transfer of electrons from one atom to the other atoms is an ionic bond.
A compound that has ionic bonds is an ionic compound. Usually, metal atoms lose electrons and become cations, and nonmetal atoms gain electrons to become anions. The electrostatic attraction between the opposite charges holds the ions together in the ionic compound. For example, sodium (Na) loses one electron, and fluorine (F) gains one electron to make a compound sodium fluoride (NaF), as illustrated in Fig. 3.1.4.
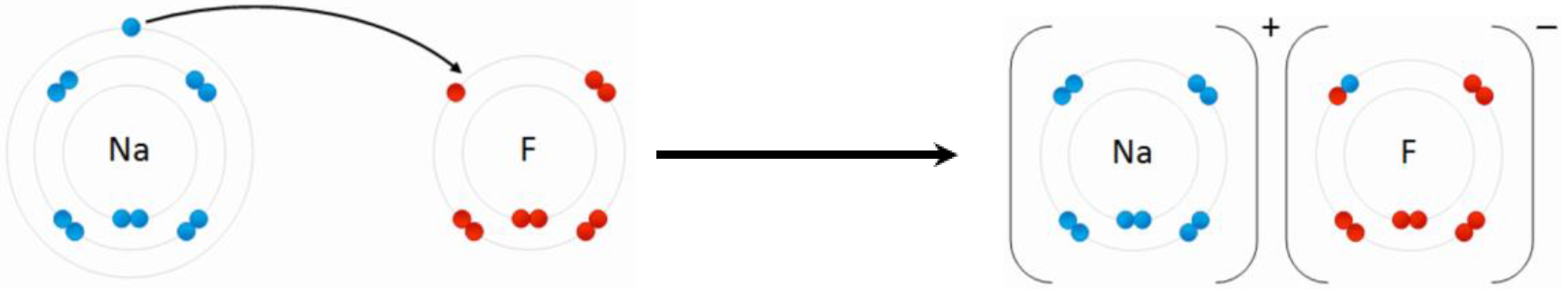
Ionic compounds
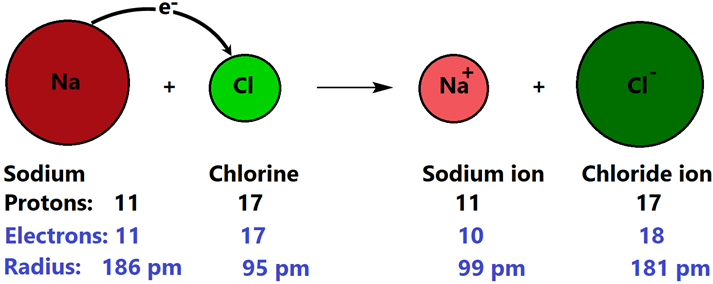
Table salt, i.e., NaCl is an example of an ionic compound. The Na completes its octet by losing one electron and becoming Na+ cation. Losing electrons reduces the electron-electron repulsion, but the electron-nucleus attraction remains the same. Consequently, the electron cloud around the nucleus shrinks. Similarly, the chlorine atom has seven valence electrons. After gaining one electron, it becomes Cl- anion with its octet complete. Gaining electrons increases the electron-electron repulsion, but the electron-nucleus attraction remains the same. Consequently, the electron cloud around the nucleus expands. Fig. 3.1.5 illustrates the formation of an ionic bond and the accompanying changes in the total number of electrons and sizes relative to the parent neutral atoms in the case of NaCl formation.
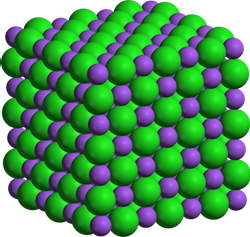
The ionic bond is not localized or unidirectional. The electrostatic force is all around the ions. Therefore, the cations surround the anions, and the anion surrounds the cations in a regular array in a 3D crystal lattice. Fig 3.1.6 illustrates the structure of NaCl.
- The formula of the ionic compounds represents the simplest whole-number ratio of the atoms of the constituent elements.
- A cation is always smaller in size than its parent neutral atom.
- An anion is always larger than its parent neutral atom.

Usually, a metal and a nonmetal bond is ionic, and the bond between two nonmetals is covalent. Better criteria are based on the difference in electronegativities of the bonded atoms. Electronegativity is the ability of an atom to attract a pair of bonded electrons to itself. If the electronegativity difference is significant, the bonding electrons completely transfer to the more electronegative atom, and the bond is ionic. There is no single value of electronegativity difference to separate ionic and covalent bonds, but usually, the electronegativity difference of more than 1.8 results in an ionic bond. Otherwise, a covalent bond, but the bonding electrons are more towards the more electronegative atom, making it a polar covalent bond. An electronegativity difference less than 0.5 is considered a noncovalent bond, but a true noncovalent bond forms when the bonded atoms are the same element.
Properties of compounds
The properties of the compounds are usually altogether different from the properties of their constituent elements. For example, hydrogen (H2) is a gas that burns in oxygen, oxygen (O2) is a gas that assists combustion, but water (H2O) is a liquid that extinguishes fire. Similarly, sodium (Na) is a soft metal that melts at 97.79 oC, chlorine (Cl2) is yellowish color gas, but sodium chloride (NaCl) is a transparent crystal that melts at 801 oC.
The intermolecular interactions in covalent molecules are weak to moderate relative to the strength of covalent bonds or ionic bonds. Therefore, the covalent molecules are usually gases like O2, NH3, CH4, liquids like H2O, or soft and low melting solids like waxes, glucose (C6H12O6, mp 146oC).
Ions in the ionic compounds are held together by strong ionic bonds in a 3D array of crystal lattices. Therefore, ionic compounds are usually hard solids with high melting points. For example, NaCl melts at 801 oC. Covalent compounds that exist as a 3D network of covalent bonds, i.e., as giant molecules, are usually hard materials having a higher melting point than ionic compounds. For example, SiO2 present in sand and quartz is a hard solid that melts at 1,710 oC. Diamond –the hardest known substance, is a giant molecule of carbon atoms held together in a 3D network of covalent bonds that melts around 4,027 oC.


