3.2: Naming binary ionic compounds
- Page ID
- 372890
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Binary ionic compounds are compounds composed of monoatomic cations and monoatomic anions. For example, NaCl is a binary ionic compound composed of monoatomic cations Na+ and monoatomic anions Cl-. Another example is CaCl2 composed of monoatomic cations Ca2+ and monoatomic anions Cl-.
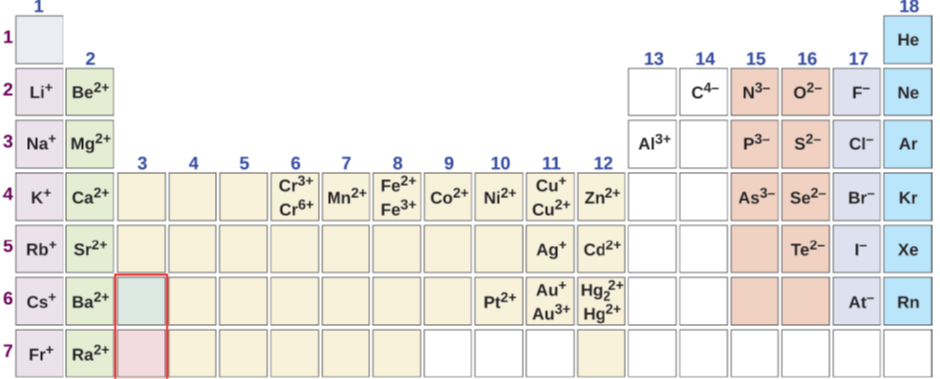
Charge on monoatomic ions
Nonmetals and metalloids of group 14 to group 17 usually form monoatomic anions. The charge on the anions is equal to the group number minus eighteen. For example, halogens in group 17 have charge: 17-18 = -1, oxygen in group 16 has charge: 16-18 = -2, and nitrogen in group 15 has charge: 15-18 = -3. Metals usually form cations: metals of group 1 from +1, metals of group 2 form +2, and aluminum of group 13 form +3 charge on cations, as shown in Fig. 3.2.1. Other metals have variable charges in compounds. The charge of the metals having variable charge can be calculated from the compound's chemical formula because the total –ve charge should be equal to the total +ve charge to make the compound neutral.
calculate the charge of iron ion in FeCl2?
Solution
Three are two chloride anions, each with a -1 charge, making a total of -2. So the charge on cation has to be +2 to balance the negative charge. Answer: Fe2+.
Calculate the charge on an iron ion in Fe2O3?
Solution
There are three oxygen anions, each with a -2 charge, making -6. So the total charge on two iron atoms should be +6, i.e., the charge on iron atoms is +3. Answer: Fe3+.
Names of monoatomic ions
Name of a monoatomic anion
The name of a monoatomic anion is the element's name with the last syllable replaced with –ide ion. For example, Cl- is a chloride ion, O2- is an oxide ion, N3- is a nitride ion, S2- is sulfide ion, and C4- is a carbide ion, derived from the element names chlorine, oxygen, nitrogen, sulfur, and carbon, respectively.
Name of a monoatomic cation having a fixed charge
Alkali metals have +1, alkaline earth metals have +2, and aluminum has +3 charge. Their name is the name of the element ending with ion. For example, Na+ is a sodium ion, Ca2+ is a calcium ion, and Al3+ is an aluminum ion.
Name of a monoatomic cation having a variable charge
The names of the cations that have a variable charge is the name of the element followed by charge in roman numeral enclosed in small bracts and ending with ion. For example, Fe2+ is an iron(II) ion, and Fe3+ is an iron(III) ion, Cu+ is a copper(I) ion, and Cu2+ is a copper(II) ion.
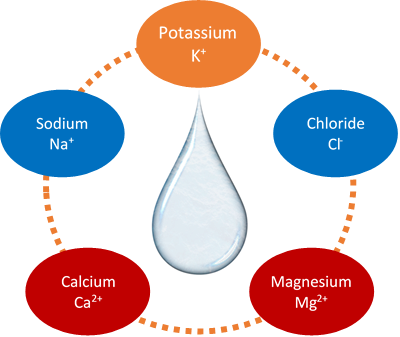
The ions that are important in body fluids include sodium ion (Na+), potassium ion (K+), calcium ion (Ca2+), magnesium ion (Mg2+), and chloride ion (Cl-), as shown in Fig. 3.2.2. Na+ is present in fluids inside the cells. It regulates and controls body fluids. K+ is present in fluids outside the cells and regulates body fluids and cell functions. Ca2+ and Ma2+ are present in the body fluids outside the cells, where Ca2+ is needed for muscle contraction, Mg2+ is needed for muscle contraction, nerve control, and enzymes. Cl- is primarily present to balance the charge of the cations in the body fluids.
Writing the formula of a binary ionic compounds
The formula of an ionic compound is the symbol of the cation element with a subscript number followed by the symbol of the anion element with a subscript number. The formula shows the simplest whole-number ratio of the constituent elements in the subscripts, such that the total positive charge is equal to the total negative charge.script on the right of the symbols tell the number if they're more than one atom of the element.
The rules are illustrated in Fig. 3.2.3:
- Write cation followed by anion with charges,
- swap the charge as a subscript of the opposite ion,
- simplify the subscript to the simplest whole-number ratio,
- use the simplified subscript in the final formula, and
- do not write the subscript if it is one.

Writing the names of an ionic compound from the formula
Writing the name of ionic compounds of cations with fixed charge
If the cation has a fixed charge in compounds, the name starts with the name of the element of the cation, followed by the name of the anion without the world ion at the end. For example, KI is potassium iodide, and CaCl2 is calcium chloride.
Writing the name of ionic compounds of cations with variable charge
Write the name of the cation, including the charge in roman numerals enclosed in small brackets but without the word ion at the end, followed by the name of the anion, without ion at the end. For example, FeCl2 is iron(II) chloride, and Fe2O3 is iron(III) oxide. Additional examples are given in Table 1.
Silver, zinc, and cadmium cations have fixed changes: Ag+, Zn2+, and Cd2+. Names of these cations are the names of the element with or without charge shown in roman numerals, both ways it is correct.
| Example# | Formula | Name of the cation | Name of the anion | Name of the compound |
|---|---|---|---|---|
| 1 | NaCl | Sodium ion | Chloride ion | Sodium chloride |
| 2 | Al2O3 | Aluminum ion | Oxide ion | Aluminum oxide |
| 3 | FeCl3 | Iron(III) ion | Chloride ion | Iron(III) chloride |
| 4 | CuO | Copper(II) ion | Oxide ion | Copper(II) oxide |
| 5 | AgCl |
Silver(I) ion, or Silver ion |
Chloride ion |
Silver(I) chloride, or Silver chloride |


