3.9: Intramolecular forces and intermolecular forces
- Page ID
- 372897
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
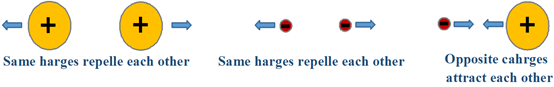
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)There are electrostatic interaction between charges or partial charges, i.e., the same charges repel each other, and opposite charges attract each other, as illustrated in Fig. 3.9.1.There are two types of electrostatic forces in compounds or molecules, intramolecular forces that exist between the bonded atoms of a compound or a molecule, and intermolecular forces that exist between molecules as described below.
Intramolecular forces
Intramolecular forces are the chemical bonds holding the atoms together in the molecules. The three major types of chemical bonds are the metallic bond, the ionic bond, and the covalent bond.
Metallic bond
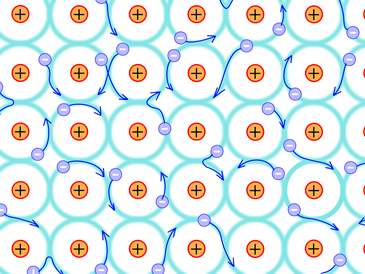
Metals exist as a collection of many atoms as +ions arranged in a well-defined 3D arrangement called crystal lattice with some of the outermost electrons roaming around in the whole piece of the metal, forming a sea of electrons around the metal atoms, as illustrated in Fig. 3.9.2. The attraction between +ions and the sea of free moving electrons is the metallic bond that holds the atoms together in a piece of metal. The metallic bond is usually the strongest type of chemical bond.
Ionic bond
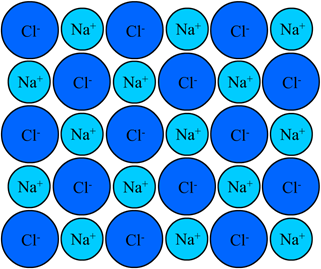
When the electronegativity difference between bonded atoms is large, i.e., more than 1.9 in most cases, the bonding electrons completely transfer from a more electropositive atom to a more electronegative atom creating a cation and an anion, respectively. There is the electrostatic interaction between cation and anion, i.e., the same charges attract each other, and opposite charges repel each other, as illustrated in Fig. 3.9.1. The cations and anions orient themselves in a 3D crystal lattice in such a way that attractive interactions maximize and the repulsive interactions minimize, as illustrated in Fig. 3.9.3. Ionic bonds are usually weaker than metallic bonds but stronger there the other types of bonds.
Covalent bond
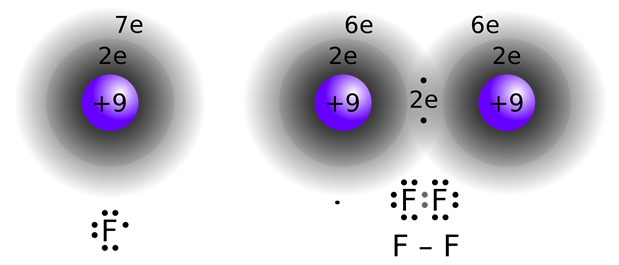
When the electronegativity difference between bonded atoms is moderate to zero, i.e., usually less than 1.9, the bonding electrons are shared between the bonded atoms, as illustrated in Fig. 3.9.4. The attractive force between the bonding electrons and the nuclei is the covalent bond that holds the atoms together in the molecules. The covalent bond is usually weaker than the metallic and the ionic bonds but much stronger than the intermolecular forces.
Criteria to predict the type of chemical bond
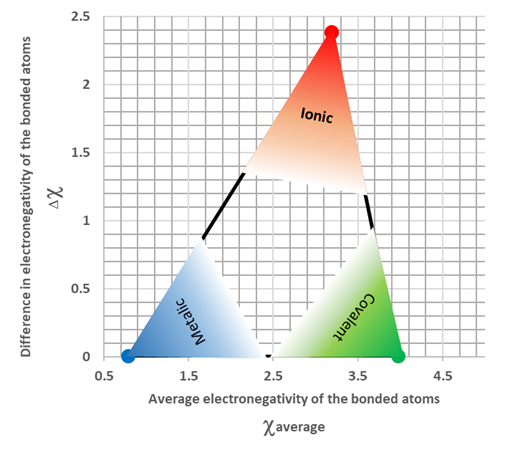
Metals tend to have lower electronegativity and nonmetals have higher electronegativity. When the electronegativity difference between the bonded atoms is large, usually more than 1.9, the bond is ionic. Generally, a bond between a metal and a nonmetal is ionic. When the electronegativity difference is low, usually less than 1.9, the bond is either metallic or covalent. Nonmetals tend to make a covalent bond with each other. Nonmetals also have higher electronegativities. So, when the average electronegativity of the bonded atom is high and the electronegativity difference between them is low, they tend to make a covalent bond. Metals tend to make the metallic bond with each other. Metals also tend to have lower electronegativity values. So, when the average electronegativity of the bonded atom is low and the electronegativity difference between them is also low, they tend to make a metallic bond. Fig. 3.9.5 illustrates the criteria to predict the type of chemical bond based on the electronegativity difference. Keep in mind that there is no sharp boundary between metallic, ionic, and covalent bonds based on the electronegativity differences or the average electronegativity values.
Intermolecular forces
Intermolecular forces are the electrostatic interactions between molecules. The intermolecular forces are usually much weaker than the intramolecular forces, but still, they play important role in determining the properties of the compounds. The major intermolecular forces include dipole-dipole interaction, hydrogen bonding, and London dispersion forces.
Dipole-dipole interactions
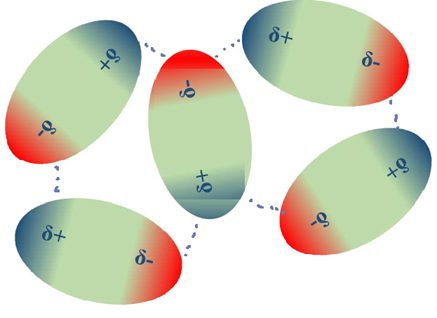
Polar molecules have permanent dipoles, one end of the molecule is partial positive (δ+) and the other is partial negative (δ-). The polar molecules have electrostatic interactions with each other through their δ+ and δ- ends called dipole-dipole interactions, though these interactions are weaker than ionic bonds. The polar molecules orient in a way to maximize the attractive forces between the opposite charges and minimize the repulsive forces between the same charges, as illustrated in Fig. 3.9.6.
Hydrogen bonds
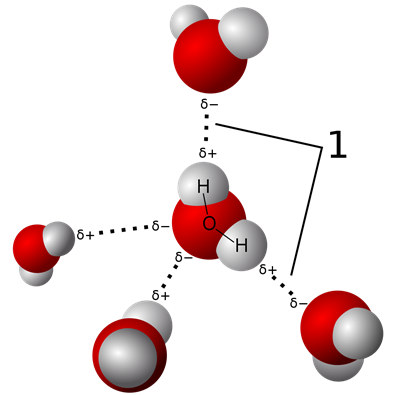
Hydrogen bonding is a dipole-dipole interaction when the dipole is a hydrogen bond to O, N, or F, e.g. in water molecules as illustrated in Fig. 3.9.7. Although hydrogen bond is a dipole-dipole interaction, it is distinguished from the usual dipole-dipole interactions because of the following special features.
- The electronegativity difference between H and O, N, or F is usually more than other polar bonds.
- The charge density on hydrogen is higher than the δ+ ends of the rest of the dipoles because of the smaller size of hydrogen.
- The δ+ Hydrogen can penetrate in less accessible spaces to interact with the δ- O, N, or F of the other molecule because of its small size.
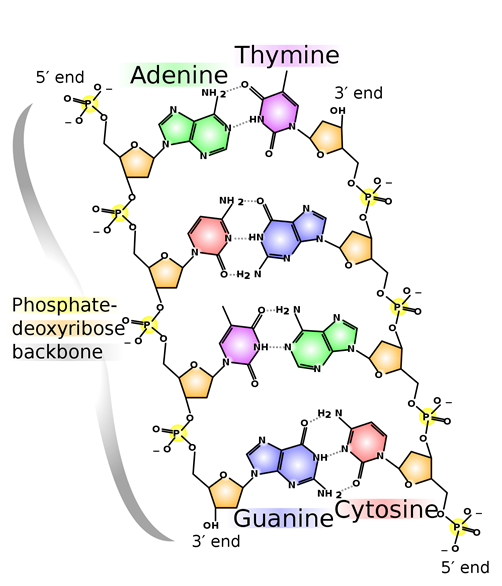
A hydrogen bond is usually stronger than the usual dipole-dipole interactions. Hydrogen bonding is the most common and essential intermolecular interaction in biomolecules. For example, two strands of DNA molecules are held together through hydrogen bonding, as illustrated in Fig. 3.9.8. Proteins also acquire structural features needed for their functions mainly through hydrogen bonding.
London dispersion forces
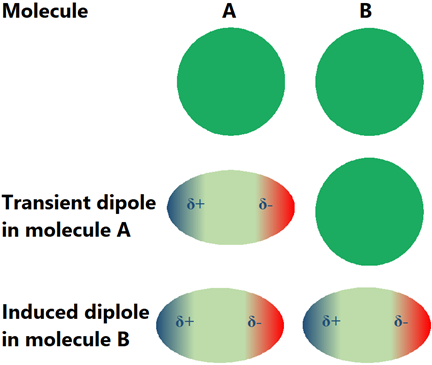
It may appear that the nonpolar molecules should not have intermolecular interactions. Practically, there are intermolecular interactions called London dispersion forces, in all the molecules, including the nonpolar molecules. The electron cloud around atoms is not all the time symmetrical around the nuclei. It temporarily sways to one side or the other, generating a transient dipole. The transient dipole induces a dipole in the neighboring. A transient dipole-induced dipole interaction, called London dispersion force or wander Wall’s force, is established between the neighboring molecules as illustrated in Fig. 3.9.9. Although London dispersion forces are transient, they keep re-appearing randomly distributed in space and time. London dispersion forces are not unique to nonpolar molecules, they are present in all types of molecules, but these are the only intramolecular forces present in the nonpolar molecules.