Oxidation State Trends in Group 4
- Page ID
- 3691
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)This page explores the oxidation states (oxidation numbers) adopted by the Group 4 elements (carbon (C), silicon (Si), germanium (Ge), tin (Sn) and lead (Pb)). It examines the increasing tendency of the elements to form compounds with +2 oxidation states, particularly for tin and lead.
Some examples of the trends in oxidation states
The typical oxidation state adopted by elements in Group 4 is +4, as in CCl4, SiCl4 and SnO2.
CH4, however, is not an example of carbon with an oxidation state of +4. Because carbon is more electronegative than hydrogen, its oxidation state is -4.
However, down the group, there are more examples of +2 oxidation states, such as SnCl2, PbO, and Pb2+. Tin's +4 state of is still more stable than its +2 state, but for lead and heavier elements, the +2 state is the more stable; it dominates the chemistry of lead.
An example from carbon chemistry
The only common example of carbon in a +2 oxidation state is carbon monoxide, CO. Carbon monoxide is a strong reducing agent because it is easily oxidized to carbon dioxide, which has a more thermodynamically stable oxidation state of +4. For example, carbon monoxide reduces many hot metal oxides to elemental metals; this reaction has many useful applications, one of which is the extraction of iron in a blast furnace.
![]()

Examples from tin chemistry
For tin and below, the +2 state is increasingly common, and there is a variety of both tin(II) and tin(IV) compounds. However, tin(IV) is the more stable oxidation state; it is therefore fairly easy to convert tin(II) compounds into tin(IV) compounds. This is best illustrated in that Sn2+ ions in solution are strong reducing agents.
A solution containing tin(II) ions (solvated tin(II) chloride, for example) reduces iodine to iodide ions. In the process, the tin(II) ions are oxidized to tin(IV) ions.
![]()

Tin(II) ions also reduce iron(III) ions to iron(II) ions: tin(II) chloride reduces iron(III) chloride to iron(II) chloride in solution. In the process, the tin(II) ions are oxidized to the more stable tin(IV) ions.
![]()
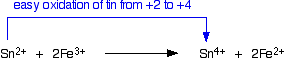
In addition, tin(II) ions are easily oxidized by powerful oxidizing agents like acidified potassium manganate(VII) (potassium permanganate). This reaction is used in a titration determination of the concentration of tin(II) ions in solution.
![]()

As a final example, in organic chemistry, tin and concentrated hydrochloric acid are traditionally used to reduce nitrobenzene to phenylamine (aniline). Tin is first oxidized to tin(II) ions and then further to preferred tin(IV) ions.
Examples from lead chemistry
With lead, the situation is reversed. The lead(II) oxidation state is the more stable; there is a strong tendency for lead(IV) compounds to react, forming lead(II) compounds. Lead(IV) chloride, for example, decomposes at room temperature to give lead(II) chloride and chlorine gas:
![]()
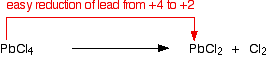
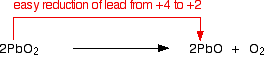
Lead(IV) oxide decomposes on heating to give lead(II) oxide and oxygen:
![]()
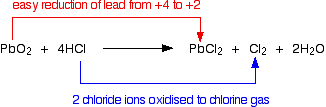
Lead(IV) oxide also reacts with concentrated hydrochloric acid, oxidizing chloride ions in the acid to chlorine gas. Once again, lead is reduced from the +4 to the more stable +2 state.
![]()
An explanation for the trends in oxidation states
There is nothing unusual about the stability of the +4 oxidation state in Group 4. Each of the elements in the group has the outer electronic structure ns2npx1npy1, where n is the period number, varying from 2 (for carbon) to 6 (for lead). In an oxidation state of +4, all valence electrons are directly involved in bonding.
Closer to the bottom of the group, there is an increasing tendency for the s2 pair to be uninvolved in bonding. This is often known as the inert pair effect, and is dominant in lead chemistry. There are two different explanations for this, depending on whether the formation of ionic or covalent bonds is in question.
The inert pair effect in the formation of ionic bonds
If the elements in Group 4 form 2+ ions, they lose their p electrons, leaving the s2 pair unused. For example, to form a lead(II) ion, lead loses its two 6p electrons, but the 6s electrons are left unchanged, an "inert pair".
Ionization energies usually decrease down a group as electrons get further from the nucleus. This is not the case in Group 4. This first chart shows how the total ionization energy needed to form a 2+ ion varies down the group. Values are given in kJ mol-1.
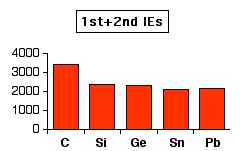
Notice the slight increase between tin and lead. This indicates that it is more difficult to remove the p electrons from lead than from tin.
However, examining the pattern for the loss of all four electrons in the chart below, this discrepancy between tin and lead is much more apparent. The relatively large increase between tin and lead is due to the greater difficulty in removing the 6s2 pair in lead than the corresponding 5s2 pair in tin.
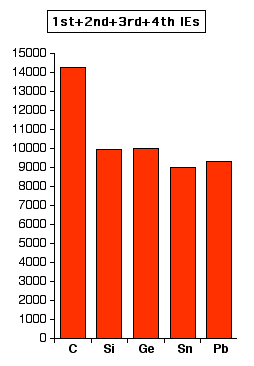
(Again, the values are all in kJ mol-1, and the two charts are on approximately the same scale.)
These effects are due to the Theory of Relativity. Heavier elements such as lead experience a relativistic contraction of the electrons that draws the electrons closer to the nucleus than expected. Because they are closer, they are more difficult to remove. The heavier the element, the greater this effect becomes. This affects s electrons to a greater degree than p electrons.
In lead, the relativistic contraction makes it energetically more difficult to remove the 6s electrons than expected. The energy releasing terms when ions are formed (like lattice enthalpy or hydration enthalpy) cannot compensate for this extra energy. Therefore, it makes no energetic sense for lead to form 4+ ions.
The inert pair effect in the formation of covalent bonds
Carbon normally forms four covalent bonds rather than two. Using the electrons-in-boxes notation, the outer electronic structure of carbon looks like this:
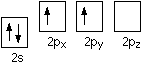
There are only two unpaired electrons. Before carbon forms bonds, however, it normally promotes an s electron to the empty p orbital.
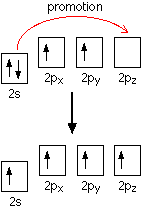
This leaves 4 unpaired electrons which (after hybridization) can go on to form 4 covalent bonds.
It is worth supplying the energy to promote the s electron, because the carbon can then form twice as many covalent bonds. Each covalent bond formed releases energy, and this is more than enough to supply the energy needed for the promotion.
One possible explanation for the reluctance of lead to do the same lies in decreasing bond energies down the group. Bond energies decrease as atoms get bigger and the bonding pair is further from the two nuclei and better screened from them.
For example, the energy released when two extra Pb-X bonds (where X is H or Cl or whatever) are formed may no longer be enough to compensate for the extra energy needed to promote a 6s electron into the empty 6p orbital. This would effect is amplified if the energy gap between the 6s and 6p orbitals is increased by the relativistic contraction of the 6s orbital.
Contributors and Attributions
Jim Clark (Chemguide.co.uk)


