Acid-Base Reactions
- Page ID
- 53177
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- Describe neutralization using chemical equations
Acids are familiar to you from everyday life, because acids are sour things, like vinegar and lemons. Bases might be less familiar to you. Common bases in regular life are baking soda and antacids that people take if they are having stomach trouble. Also, many soaps are basic, although this is just because of how they are made, not necessary to make them soapy. Bases make things feel slippery, and they taste bitter. They can be used to clean greasy things. Common acids and bases will react with each other and when they react completely, the products are usually a salt solution that isn't sour or bitter. This is called neutralization. Acid-base neutralization reactions are what make most cakes fluffy, because sometimes these reactions generate a gas that makes holes in the cake.
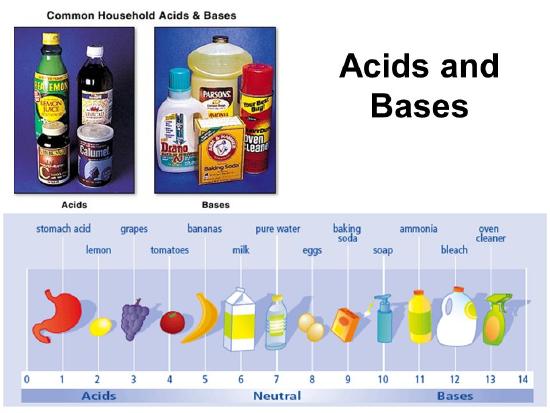
It is important to know that there are many different definitions of acid and base. This page describes the simplest and most specific definition. Practicing chemists use broader definitions that stretch the general concept to a variety of situations that are important in more advanced chemistry.
How does Neutralization work?
This description describes acid-base reactions in water. (It works a little bit differently in other solvents, but you don't need to think about that too much until you study more advanced chemistry). An acid is an electrolyte (strong or weak) that produces H+ ions when it dissolves in water. Hydrogen ions are also called protons, because a hydrogen nucleus is just a proton (unless it is a heavier isotope, but this is rare). Acids are sometimes called "proton donors" meaning they give away protons, but this is not a very good word, because the protons are pulled away by the solvent, not dropped by the solute. Examples of strong acids include HCl and H2SO4, which are called hydrochloric acid and sulfuric acid. But sulfuric acid has 2 protons bound to sulfate ion, and only one comes off completely (just like the weak electrolytes discussed here), so a solution of sulfuric acid will have hydrogen ions, bisulfate ions, and sulfate ions in solution. Just like in the case of precipitation reactions, if a base is added, both protons might come off completely and react with the base. Acids are called monoprotic, diprotic, etc. depending on how many acidic protons they have. HCl, acetic acid (vinegar, CH3COOH) and nitric acid (HNO3) are monoprotic acids. (Acetic acid has other protons, but only the last one is acidic.) Sulfuric acid and many others are diprotic acids.

A base is an electrolyte (strong or weak) that produces hydroxide ions when dissolved in water solution. This could be because it is a hydroxide salt, like NaOH, or because it takes hydrogen ions from water, leaving hydroxide behind. A good example of this is ammonia, NH3, which is sometimes used in house cleaning products. Ammonia reacts with water to make ammonium hydroxide (but only a little bit, ~1% of the ammonia reacts):
\[NH_{3}(aq) + H_{2}O(l) \rightarrow NH_{4}^{+}(aq) + OH^{–}(aq)\]
In general, bases react with hydrogen ions. This is how neutralization happens. The acid produces hydrogen ions, and the base produces hydroxide ions. These react together to make water. The anion that came from the acid and the cation are left, so if you evaporate the water, you would get a salt. The general reaction looks like this:
\[X^{–}(aq) + H^{+}(aq) + M^{+}(aq) + OH^{–}(aq) \rightarrow H_{2}O(l) + X^{–}(aq) + M^{+}(aq)\]
Overall, the reaction is:
\[H^{+}(aq) + OH^{–}(aq) \rightarrow H_{2}O(l)\]
Thus, the hydrogen ions, which makes acids acidic, are consumed, and the hydroxide which makes bases basic is also consumed, and if the moles of acid and base are equal, only neutral water and a salt is left. (Actually, it is a little bit more complicated than this if the acid or base is weak. The solution will only really become neutral when the moles are equal if both are strong.)
Need to Know Acids and Bases
| Name | Formula | Comment |
|---|---|---|
| Ammonia | NH3 | weak base |
| Alkali Metal Hydroxides | LiOH, NaOH, KOH, RbOH, CsOH | soluble strong bases |
| Alkaline Earth Metal Hydroxides | Ca(OH)2, Sr(OH)2, Ba(OH)2 | soluble strong bases |
| Acetic Acid | HC2H3O2 | vinegar, weak acid |
| Perchloric Acid | HClO4 | strong acid |
| Chloric Acid | HClO3 | strong acid |
| Nitric Acid | HNO3 | strong acid |
| Carbonic Acid | H2CO3 | a weak acid, in sodas |
| Bicarbonate | HCO3– | a weak base (baking soda) |
| Sulfuric acid | H2SO4 | diprotic strong acid |
| Bisulfate | HSO4– | weak acid |
| Hydrohalic Acids | HCl, HBr, HI | strong acids |
| Hydrofluoric Acid | HF | weak acid, but very dangerous |
Example
Q: How do acid-base reactions make cake fluffy?
A: Usually cakes include an acidic ingredient (this varies) and sodium bicarbonate, a base. When they react, the proton from the acid is transferred to the bicarbonate, making the weak acid carbonic acid. Carbonic acid is the product of an acid anhydride reaction between carbon dioxide and water. This reaction can be reversed, or carbonic acid can decompose into water and carbon dioxide. Especially at the high temperatures inside a baking cake, this decomposition will happen, and produce carbon dioxide gas. The pressure of the hot gas will form bubbles inside the cake, making it fluffy.
In the previous section (Precipitation), instead of having hydroxide react with hydrogen ions to form water, the acid base reaction made carbonic acid from protons and bicarbonate. In general, a base is something that will bind tightly to a proton. Bicarbonate and carbonate ions are bases, and so are sulfide ions. Both of these reactions can produce a gas, either carbon dioxide or hydrogen sulfide. In the lab, sodium bicarbonate is usually used to neutralize acid spills. When it reacts with acid it produces bubbles, so it's easy to see when the reaction finishes.
Most chemists agree that acid-base reactions are combination reactions without redox. This is a much more general definition than described here, but after you read about redox, go over these examples and convince yourself that they all fit that definition. What other examples can you think of that also fit this definition?
Summary
Acids are strong/weak electrolytes that produce H+ ions when dissolved in water. A notable property of acids is that they have a sour taste. Bases are strong/weak electrolytes that produce hydroxide ions (OH-) when dissolved in water. They are also known to have a bitter taste as well as a slippery or soapy texture. Neutralization is a type of reaction in which acids react with bases to form a salt solution (water and a salt), usually.
Outside Links
- CrashCourse Chemistry: Acid-Base Reactions in Solution (11 min)
- Khan Academy: Acid Base Introduction
(18 min, skip the last 3 min, optional: skip the first 4 min)
Contributors and Attributions
Emily V Eames (City College of San Francisco)

