HW Solutions #7
- Page ID
- 2850
1. Why can carbon only form 4 bonds when elements in the rest of the group can form up to six? Carbon is a relatively small atom. There is physically not enough room for 6 other atoms to bind to carbon. Also, other elements in Carbon's group bond to 6 other atoms with an expanded octet. Due to its electron configuration, carbon does not have the ability to expand the octet.
2. What is the “inert pair effect”? The inert pair effect is the tendency for s-orbital electrons in D and F block metals to abstain from participation in chemical bonding.
3. Write the formulas for three different nitrogen oxides
NO, NO2, N2O
4. Which elements form ionic hydrides? Why?
Ionic hydrides are usually formed by very electropositive elements. Alkali group elements are very electropositive. The hydride ion will take the electron from the electropositive atom, and both species will be left with a more stable electron configuration.
Sean Gottlieb
5. Which element has the highest melting point, sodium or potassium? Why?
Sodium is smaller than potassium, and so will exhibit more intermolecular attraction. So, sodium will be more difficult to melt --> higher melting point.
Sean Gottlieb
6. Which element has the highest melting point, chlorine or bromine? Why?
7. Which do you expect to have the larger atomic radius, carbon or nitrogen?
Carbon will have the larger atomic radius.
Sean Gottlieb
8. Write possible chemical equations for the following elements reaction with Cl2:
- Rubidium: 2Rb(s) + Cl2(g) --> 2RbCl(s)
- Calcium: Ca(s) + Cl2(g) --> CaCl2(s)
- Lead: Pb(s) + Cl2(g) --> PbCl2(s)
- Carbon: CH4(g) + 4Cl2(g) --> CCl4(l) + 4HCl(g)
Sean Gottlieb
9. What are the most common oxidation states for the following elements: (each element may have more than one)
- Boron +3
- Oxygen -2
- Iodide -1, +5
- Cesium +1
- Sodium +1
- Nitrogen -3, +1, +2, +4, +5
- Lead +2, +4
- Magnesium +2
--- Hao
10. Which of the following elements commonly exist in oxidation state +1?
- Lithium
- Magnesium
- Yttrium
- Manganese
- Copper
- Mercury
- Boron
- Indium
- Nitrogen
- Oxygen
- Chlorine
11. Which do you expect to have more metallic character, tin or lead?
Lead is expected to have more metallic character, because generally electronegativity decreases when going down along the same group in periodic table. However, in fact, tin and lead are rather similar to each other, having comparable first ionization energies and standard electrode potentials.
--- Hao
12. Name at least two allotropes of carbon
graphite and diamond
--- Hao
13. Explain why CO2 is a gas while SiO2 is a solid
The intermolecular forces between CO2 molecules are mainly van der Waals forces, which are relatively weak. In contrast, in SiO2, each Si atom is bonded to four O atoms and each O atom to two Si atoms. Thus, the structure of SiO2 is that of a network covalent solid, which is expected to have a much higher boiling point compared to CO2. That is why CO2 is a gas and SiO2 is a solid at ambient conditions.
--- Hao
14. Which one is the strongest reducing agent, Mg or Ba?
Reducing agent gets oxidized. The most likely element to get oxidized is the one that is the least electronegative, or more electropositive. This element will be more likely to give up its electrons, thus getting oxidized. Generically, the trend of increasing strength of a reducing agent will be the opposite of the increasing electronegativity trend on the periodic table. Ba is further down than Mg, so it will be the stronger reducing agent.
~Rachel
15. Which one is the strongest oxidizing agent, Cl2 or Br2?
16. Why can phosphorous form up to 6 covalent bonds when nitrogen can only form four?
Electron configurations:
N: 1s22s22p3 P: 1s22s22p63s23p3
The valence electrons in N are in the n=2 shell, whereas the valence electrons in P are in the n=3 shell. In order to have a full shell, N can accept 3 more electrons. That is why N normally forms three bonds as in NH3. However, four bonds can be formed as in NH4+ if one of N's valence electrons is initially removed. For P, the n=3 shell can hold a total of 18 electrons in the 3s, 3p, and 3d orbitals. This is why P can have more bonds, since electrons can also enter the 3d orbitals. Hybridization is also good to remember here, in that P with six bonds uses the sp3d2 hybridized orbitals and N uses sp3 hybridized orbitals. Technically, P could form many more than 6 bonds since it can accept 13 extra electrons, but also remember that the size of the atom can also affect how many bonds are formed.
~Rachel
18. Write equations for each of the alkali metals reacting with (excess) oxygen
The alkali metals tarnish in air due to the formation of an oxide or hydroxide on the surface. Alkali metals when burnt in air form different kinds of oxides. For example the alkali metals on reaction with limited quantity of oxygen form normal oxides of formula, M2O
4M + O2 → 2M2O (Where M = Li, Na, K, Rb, Cs)
All alkali metals will form oxides in the form of M2O, but only lithium will form this normal oxide in excess oxygen:
4 Li(s) + O2 (g) 2 Li2O(s)
When heated with excess of air, lithium forms normal oxide,Li2O ; sodium forms peroxide, Na2O2, whereas Potassium, Rubidium and Cesium react with oxygen to form
super-oxides with the general formula of MO2 for example potassium:K(s) + O2 (g) KO2 (S)
4Li + O2 → 2Li2O ( Lithium oxide)
K + O2 → KO2 ( Potassium Superoxide)
19. Complete and balance the following reactions: (If no reaction occurs state so)
- Li (s) + H2O (l) → Li+(aq) + OH-(aq) + H2(g)
- N2 (g) + H2 (g) → No rxn
- C (s) + O2 (g) → CO2(g)
- 2Cs (s) + H2 (g) → 2CsH(s)
- Sr (s) + 2H2O (l) → Sr(OH)2(aq) +H2(g)
- C (s) + H2 (g) → No rxn
- H2 (g) + Cl2 (g) → 2HCl(g)
- CO2 (g) + H2O (l) → H2CO3(aq)
- 2K (s) + Br2 (l) → 2KBr(aq)
~Rachel
20. What is a metallic hydride?
Metallic hydrides are formed by heating hydrogen gas with their metals or their alloys.
A compound in which hydrogen is bonded chemically to a metal or metalloid element. The compounds are classified generally as ionic, transition metal, and covalent hydrides. Covalent hydrides are of two subtypes, binary and complex. Certain hydrides have achieved a position of modest industrial importance, but most are of theoretical interest only.
21. Why does phosphorous have several different allotropes while nitrogen only exists as N2 (g)?
All nitrogen compounds are ultimately derived N2 and are usually stable. Thus, N2 has limited reactivity based on its electronic structure. The bond between two N atoms in N2 is a triple covalent bond. Triple covalent bonds are strong and hard to break. The change in enthalpy associated with breaking one mole of N2 bond is highly endothermic (requires energy to break bond, see example below) and the Gibbs energy of formation is positive (the reaction of formation is non-spontaneous). Therefore, nitrogen only exist as N2.
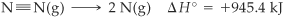
Phosphorous have several different allotropes. Two of them are often referred to as white phosphorous and red phosphorous. White phosphorous is a white, waxy, phosphorescent solid and has a P4 basic structural unit with a tetrahedral structure (figure below). The phosphorous to phosphorous (P-P) in P4 is involved in the overlap of the 3p orbitals. The overlap usually produces a 90o bond angle, but produces a more strained 60o bond angle between the P-P-P angle in P4. Species with strained bonds are highly reactive.

When white phosphorous is heated to 300oC and out of air contact, the white phosphorous transforms to red phosphorous. One P-P bond per P4 molecule breaks. Each fragment join together into a long chains (Figure below). Red P is thermodynamically more stable than than white P. White P has assigned values of 0 for enthalpy and Gibbs energy. Red P is negative for enthalpy (exothermic) and Gibbs energy (spontaneous).
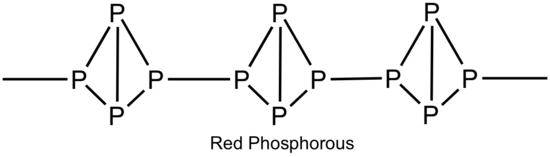
~Diana Wong
22. According to the periodic trends, which do you expect to
23. Explain why lithium is a better reducing agent than sodium
improved reactivity of lithium over sodium. Lithium has a stronger affinity for oxygen than sodium. the difference in reducing power is due to electropositive nature of Li
24. Which element is more likely to be found in oxidation state +3, phosphorous or bismuth?
25. How do the melting points of the noble gases compare to those of the rest of the periodic system? Why?
The melting points of noble gases are lower than those of the rest of the periodic system. Noble gases are chemically inert meaning that they have the inability to form bonds with other atoms (with some exceptions). Their chemical inertness is the reason why the melting points of noble gases are so low. The noble gases exist as single atoms and lack dipole moments, only weak van-der-waals interactions are present to hold the noble gas atoms together. As a result very little thermal energy is required to disrupt these weak forces which is the reason why noble gases have extremely low melting points when compared to other elements in the periodic table. Helium, for instance, exist as a liquid in temperatures approaching 0K (At 0K Helium is still a liquid at 1 atmosphere, more pressure is needed to make Helium solidify), while all other substances freeze to solid at temperatures above 0K.
~Diana Wong
26. Which element in the periodic system forms most compounds with noble gases?
The conditions necessary to form noble gas compounds as Linus Pauling predicted are (1) a readily ionizable, high atomic number noble gas atom with (2) a highly electronegative atom such as Flourine or Oxygen bonded to it.
~Diana Wong
- Have the highest ionization energy, sodium or potassium?
- Have the lowest boiling point, argon or xenon?
- Be the best reducing agent, potassium or calcium?
- Have the highest electrode potential, fluorine or chlorine?
- Have the smallest electrode potential, magnesium or strontium?
- Have the largest atomic radius, germanium or arsenic?
- Have most metallic character, indium or lead?
- Be more basic, Al2O3 or SiO2?
- Have more ionic character, In2Br6 or Ga2Br6

