HW Solutions #8
- Page ID
- 2851
1. In the following decomposition reaction,
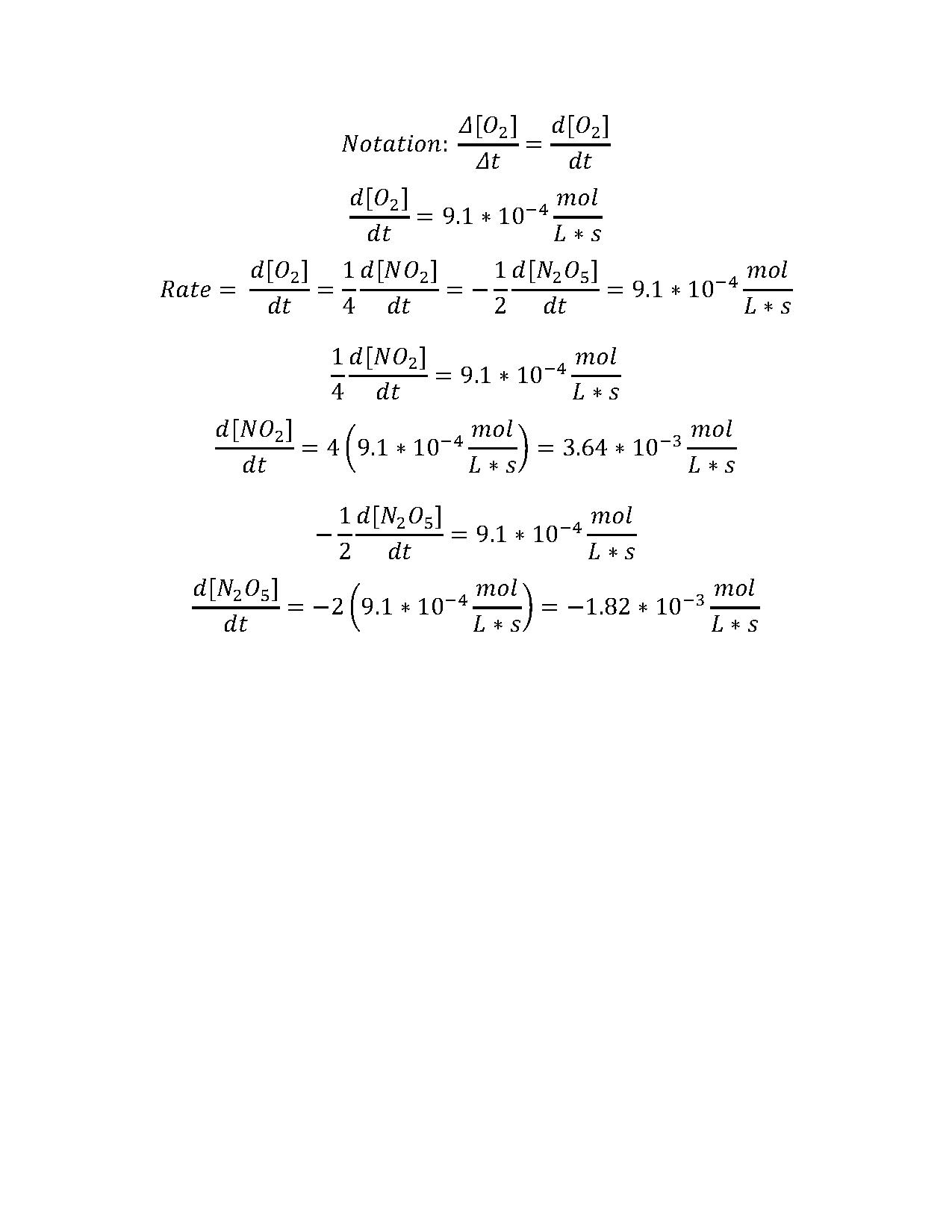
2 N2O5 → 4 NO2 + O2
oxygen gas is produced at the average rate of 9.1 × 10-4 mol · L-1 · s-1. Over the same period, what is the average rate of the following:
- the production of nitrogen dioxide
- the loss of nitrogen pentoxide
Rachel
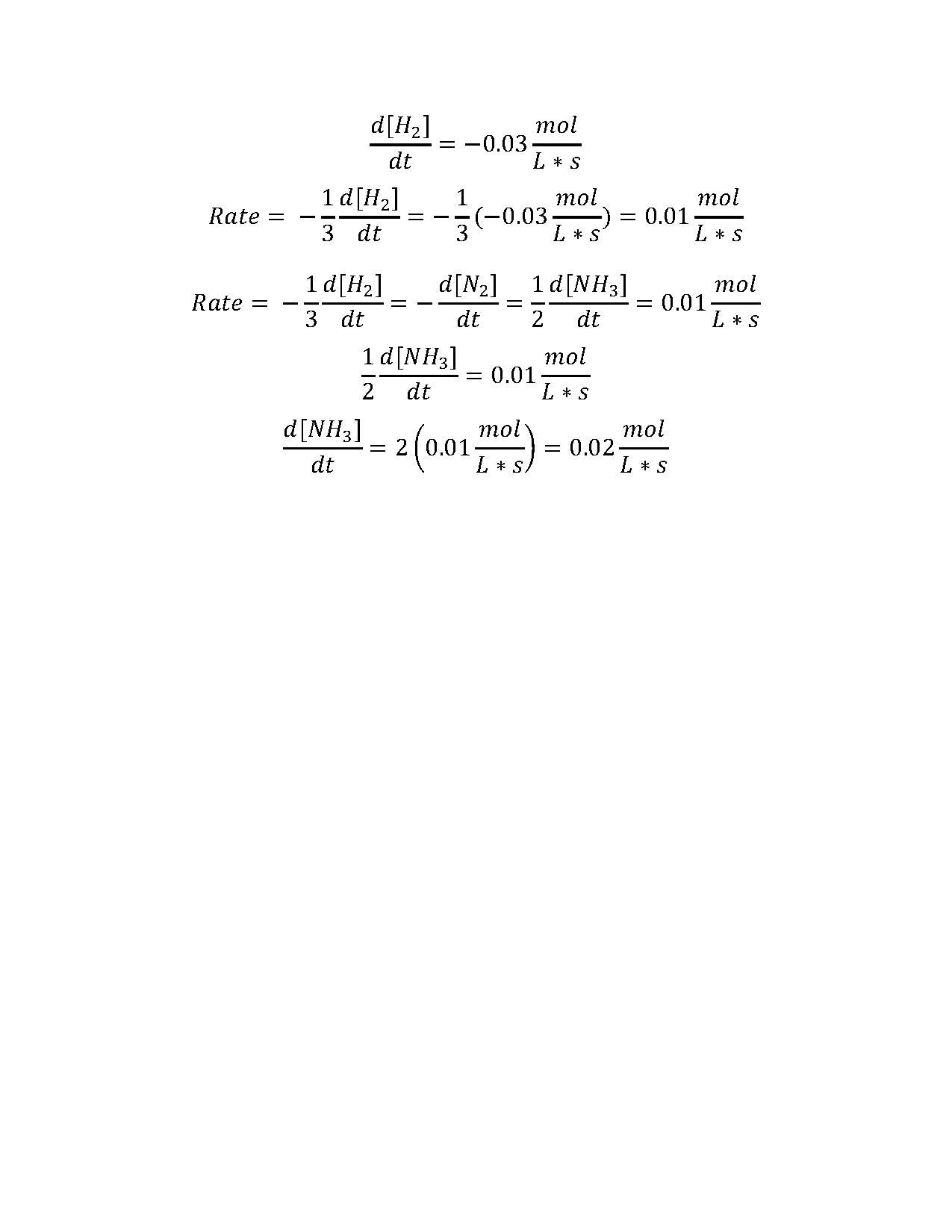
2. Consider the following reaction:
N2(g) + 3 H2(g) → 2 NH3(g)
If the rate of loss of hydrogen gas is 0.03 mol · L-1· s-1, what is the rate of production of ammonia?
Rachel
3. Nitrogen monoxide reacts with hydrogen gas to produce nitrogen gas and water vapor. The mechanism is believed to be:
| Step 1: | 2 NO → N2O2 |
| Step 2: | N2O2 + H2 → N2O + H2O |
| Step 3: | N2O + H2 → N2 + H2O |
For this reaction find the following:
- the overall balanced equation
- 2NO + 2H2 → N2 + 2H20
- any reaction intermediates
- N2O2 and N2O
4. Give two reasons why most molecular collisions do not lead to a reaction.
5. An important function for managers is to determine the rate-determining steps in their business processes. In a certain fast-food restaurant, it takes 3 minutes to cook the food, 1.5 minutes to wrap the food, and 5 minutes to take the order and make change. How would a good manager assign the work to four employees?
The restaurant chain is a consecutive process. The processes are sequential and one cannot happen in isolation. The longest time which ( 5 minutes) is the time required to take the order and give change is the rate determining step of the business. In order to be effective the manager can assign two employees to the taking of the order and assigning change in order to reduce this time and possibly attend to other customers. While the other two employees can be assigned to cooking of the food and wrapping the food. During the down times or between orders, more people can always be shifted to the RDS.
6. For the hypothetical reaction
2A + 3B → 3C + 2D
the following rate data were obtained in three experiments at the same temperature:
| Initial [A] | Initial [B] | Initial rate |
| (mole liter-1) | (mole liter-1') | (moles of A consumed liter-1‘ sec-1) |
| 0.10 | 0.10 | 0.10 |
| 0.20 | 0.10 | 0.40 |
| 0.20 | 0.20 | 0.40 |
a.) Determine the experimental rate equation for the reaction
Rate Equation predicts the relationship between the rate of reaction and the concentration of the reaction in a chemical kinetics study.
Rate of Reaction=k[A]m[B]n
Where [A] and [B] represents the reactant molarities and “m” and “n” are determined by the experiment and are not stoichiometric coefficients
To solve for “m” and “n”, first plug known values (rate, [A], and [B]) for each experiment:
Expt #1 (Rate1): 0.10 =k[0.1]m[0.1]n
Expt #2 (Rate2): 0.40 =k[0.2]m[0.1]n
Expt #3 (Rate3): 0.40 =k[0.2]m[0.2]n
Expt #1 and Expt #2 have equivalent [B] and thus, we can solve for “m” by dividing
Rate1/Rate2 = (0.1/0.4) = (k[0.1]m[0.1]n)/(k[0.2]m[0.1]n)
(0.1/0.4) = (0.1/0.2)m
0.25=(0.5)m
log(0.25)=mlog(0.5)
m=log(0.25)/log(0.5)
m=2
Expt #2 and Expt #3 have equivalent Rates (and [A]) and thus, we can solve for “n” by dividing
Rate2/Rate3 = (0.4/0.4) = (k[0.2]m[0.1]n)/(k[0.2]m[0.2]n)
1 = (0.1/0.2)n
1=(0.5)n
log(1)=nlog(0.5)
n=log(1)/log(0.5)
n=0
Plugging “m” and “n” to Rate of Reaction equation, Rate=k[A]m[B]n gives:
Rate=k[A]2[B]0
Rate=k[A]2
b. Calculate the specific rate constant, k.
Rate = k[A]2 Plug in [A] and Rate from Expt#1 to solve for k: 0.40 = k [0.2]2 k=10 L s-1moles-2 (Note: Rates and [A] from Expt #2 and #3 should generate the same “k”) c.) What is the rate of this reaction when [A] =0.30M and [B] = 0.30M?
Rate = k[A]2 Rate = (10)[0.30M]2 Rate = 0.90 L/s Diana Wong 7. For the hypothetical reaction 2A + B → 2C the following data were collected in three experiments at 25°C: 300 = k(0.10)1(0.20)2 k = 75000 OR OR - John O. 8. In the reaction 2NO + Cl2 → 2NOCl the reactants and products are gases at the temperature of the reaction. The following rate data were measured for three experiments: (a) From these data, write the rate equation for this gas reaction. What order is the reaction in NO, Cl2, and overall? If we double the initial pressure of NO, ceteris paribus, the rate is quadrupled. This means the reaction is second order for NO. If we double the pressure of Cl2, ceteris paribus, the rate is approximated doubled as well, showing a first order reaction for Cl2. The overall reaction has an order of the sum of the order of the reactants, 3. This is very similar to example 14-3 on page 579 of the text. Sean Gottlieb Knowing the orders determined from part a) we can plug and chug to solve for k: rate = k[NO]2[Cl2]1 5.1 x 10-3 = k[0.5]2[0.5] k = 0.0408 s-1 atm-2 This is similar to example 14-4 on page 580 of the text. Sean Gottlieb 9. The reaction 2NO + O2 → 2NO2 is first order in oxygen pressure and second order in the pressure of nitric oxide. Write the rate expression. Gaseous reactions are often measured in in terms of gas pressures Rate = k * p{NO}2 * p{O2}1 Diana Wong 10. The reaction A + B + C → D + F was found to be zero order with respect to A. A solution of reactants A, B, and C was prepared with the following initial concentrations: 0.2M of A, 0.4M of B, and 0.6M of G. The concentration of A in this solution dropped to essentially zero in 5 min. A second solution was prepared with the following initial concentrations: 0.03M of A, 0.4M of B, and 0.6M of C. How long will it take for A to disappear? 11. For the reaction 2NO + H2 → N2O + H2O the following experimental rate data are collected in three successive experiments at the same temperature: Using these experimental data, write the rate expression for the reaction. rate = k[NO]m[H2]n experiment 1: 0.18 molL-1s-1 = k(0.60 molL-1)m(0.37 molL-1)n experiment 2: 0.72 molL-1s-1 = k(1.20 molL-1)m(0.37 molL-1)n experiment 3: 1.44 molL-1s-1 = k(1.20 molL-1)m(0.74 molL-1)n equation 2 divided by 1: (0.72/0.18) = (1.20/0.60)m m = 2 equation 3 divided by 2: (1.44/0.72) = (0.74/0.37)n n = 1 plug into equation 1: 0.18 molL-1s-1 = k(0.60 molL-1)2(0.37 molL-1)1 solved k = 1.4 mol-2L2s-1 rate law: rate = 1.4 mol-2L2s-1[NO]2[H2] ---- Hao 12. The reaction 2HCrO4- + 3HSO3- + 5H+ → 2Cr3+ + 3SO42- + 5HQO follows the rate equation Rate = k[HCrO4-][HSO3-]2[H+] Why isn’t the rate proportional to the numbers of ions of each kind that are shown by the equation? The rate has units molarity/time. So it is not dependent on just the number of moles, but the molarities of the reactants. Also, it is important to know that the order of the reaction for each reactant is not necessarily equal to its coefficient in the chemical equation. Sean Gottlieb 13. The reaction SO2Cl2 —> SO2 + Cl2 is a first-order reaction with the rate constant k = 2.2 x 10-5 sec-1 at 320°C. What fraction of SO2Cl2 is decomposed on heating at 320°C for 90 min? for first order reaction, ln([A]o/[A]t) = kt for t = 90 min = 5400 s, ln([A]o/[A]t) = 2.2 X 10-5 s-1 X 5400 s solved [A]o/[A]t = 1.13 fraction of decomposed SO2Cl2 = 1- [A]t/[A]o = 1-(1/1.13) = 0.11 ---- Hao 14, It often is said that, near room temperature, a reaction rate doubles if the temperature is increased by 10°C. Calculate the activation energy of a reaction whose rate exactly doubles between 27°C and 37°C. k=A exp[-Ea/(R*T)] 2k=A exp[-Ea/(R*(T+10))] A exp[-Ea/(R*(T+10))]=2A exp[-Ea/(R*T)] exp[-Ea/(R*(T+10))]=2*exp[-Ea/(R*T)] -Ea/(R*(T+10))=ln(2) - Ea/(R*T) -Ea/(R*(T+10)) + Ea/(R*T)=ln(2) Ea/R [1/(T) + 1/(T+10)] = ln(2) Ea = R*ln(2)/[1/300 - 1/310]= 54 kJ/mol -Erik 15. What is the activation energy for a reaction for which an increase in temperature from 20°C to 30°C exactly triples the rate constant? 16. The following data give the temperature dependence of the rate constant for the reaction N2O4 → 2NO2 → 1/2O2. Plot the data and calculate the activation energy of the reaction. T(K) k (sec-1) Plot ln(k) versus 1/T and construct a linear fit. From the arrhenius equation: ln(k)=ln(A)-Ea/(RT) Therefore: slope=-Ea/R Ea=-slope*R=12376 (K) *8.314 (JK-1mol-1)= 102,894 J/mol ~ 103 kJ/mol -Erik 17. Consider the reaction CH4 + Cl2 → CH3Cl + HCl (occurs under light) The mechanism is a chain reaction involving Cl atoms and CH3 radicals. Which of the following steps does not terminate this chain reaction? Sean Gottlieb
Initial [A] Initial [B] Initial rate (mole liter-1) (mole liter-1) (moles of A consumed liter-1 sec-1) 0.10 0.20 300 0.30 0.40 3600
0.30 0.80 14400
R=k[A]1[B]2
Solution:
General equation: k[A]m[B]n
R1= k[A]1m[B]1n, R2= k[A]2m[B]2n, and R3= k[A]3m[B]3n , However we also know [A]3=[A]2 and [B]3=2[B]2
Substitute this into R3 to give : R3= k[A]2m(2[B]2)n
Divide the rates to find the increase: R3/R2 = 14400/3600 = 4
Now Divide the equations for R3 and R2 the same way:
R3/R2 = = (cancel like terms)
= 2n
We now have 2n = 4 and we can evaluate that n = 2 (22 = 4)
Do the same with R1 and R2 to find m:
R1= 300 = k[A]1m[B]1n = k(0.10)m(0.20)2 = k(0.10)m0.04
7500 = k(0.10)m rearranging gives k= 7500/(0.1)m
R2= 3600 = k[A]2m[B]2n = k(0.30)m(0.40)2 = k(0.30)m0.016
22500 = k(0.30)m rearranging gives k= 22500/(0.3)m
7500/(0.1)m = 22500/(0.3)m
(0.3)m/(0.1)m = 22500/7500
(0.3/0.1)m = 3
3m = 3
m = 1
R=k[A]1[B]2
- John O.
3600 = k(0.30)1(0.40)2 k = 75000
14400 = k(0.30)1(0.80)2 k = 75000
Initial p{NO} Initial p{Cl2} Initial rate
(atm) (atm) (moles of A consumed atm sec-1) 0.50 0.50 5.1 x 10-3 1.0 1.0 4.0 x 10-2
0.50 1.0 1.0 x 10-2
(b) Calculate the specific rate constant for this reaction.
Initial [NO] Initial [H2] Initial rate (mole liter-1) (mole liter-1') (moles of A consumed liter-1‘ sec-1) 0.60 0.37 0.18 1.20 0.37 0.72
1.20 0.74 1.44
273 7.87 x 10-7
298 3.46 x 10-5
308 1.35 x 10-4
318 4.98 x 10-4
328 1.50 x 10-3
338 4.87 x 10-3

