Chemistry of Fluorine (Z=9)
( \newcommand{\kernel}{\mathrm{null}\,}\)
Fluorine (F) is the first element in the Halogen group (group 17) in the periodic table. Its atomic number is 9 and its atomic weight is 19, and it's a gas at room temperature. It is the most electronegative element, given that it is the top element in the Halogen Group, and therefore is very reactive. It is a nonmetal, and is one of the few elements that can form diatomic molecules (F2). It has 5 valence electrons in the 2p level. Its electron configuration is 1s22s22p5. It will usually form the anion F- since it is extremely electronegative and a strong oxidizing agent. Fluorine is a Lewis acid in weak acid, which means that it accepts electrons when reacting. Fluorine has many isotopes, but the only stable one found in nature is F-19.
Quick Reference Table
| Symbol | F |
|---|---|
| Atomic Number | 9 |
| Group | 17 (Halogens) |
| Electron Configuration | 1s22s22p5 |
| Atomic Weight | 18.998 g |
| Density | 1.7 g/L |
| Melting Point | -219.62oC |
| Boiling Point | -188.12oC |
| Critical Point | 144.13K, 5.172 MPa |
| Oxidation States | -1 |
| Electronegativity | 3.98 |
| Stable Isotopes | F-19 |
Brief History
In the late 1600's minerals which we now know contain fluorine were used in etching glass. The discovery of the element was prompted by the search for the chemical substance which was able to attack glass (it is HF, a weak acid). The early history of the isolation and work with fluorine and hydrogen fluoride is filled with accidents since both are extremely dangerous. Eventually, electrolysis of a mixture of KF and HF (carefully ensuring that the resulting hydrogen and fluorine would not come in contact) in a platinum apparatus yielded the element.

Fluorine was discovered in 1530 by Georgius Agricola. He originally found it in the compound Fluorspar, which was used to promote the fusion of metals. It was used in this application until 1670, when Schwanhard discovered its usefulness in etching glass. Pure fluorine (from the Latin fluere, for "flow") was not isolated until 1886 by Henri Moissan, burning and even killing many scientists along the way. It has many uses today; a particular one was being used in the Manhattan project to help create the first nuclear bomb.
Electronegativity of Fluorine
Fluorine is the most electronegative element on the periodic table, which means that it is a very strong oxidizing agent and accepts other elements' electrons. Fluorine's atomic electron configuration is 1s22s22p5 (see Figure 2).
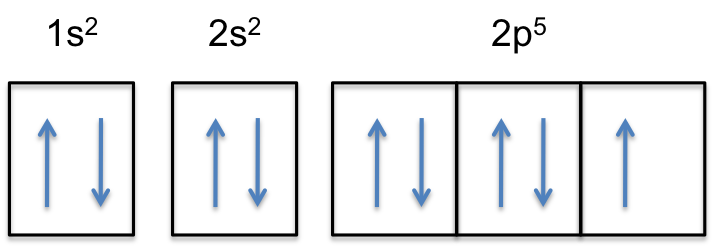
Fluorine is the most electronegative element because it has 5 electrons in its 2p shell. The optimal electron configuration of the 2p orbital contains 6 electrons, so since fluorine is so close to ideal electron configuration, the electrons are held very tightly to the nucleus. The high electronegativity of fluorine explains its small radius because the positive protons have a very strong attraction to the negative electrons, holding them closer to the nucleus than the bigger and less electronegative elements do.
Reactions of Fluorine
Because of its reactivity, elemental fluorine is never found in nature, and no other chemical element can displace fluorine from its compounds. Fluorine bonds with almost any element, both metals and nonmetals, because it is a very strong oxidizing agent. It is very unstable and reactive since it is so close to its ideal electron configuration. It forms covalent bonds with nonmetals, and since it is the most electronegative element, is always going to be the element that is reduced. It can also form a diatomic element with itself (F2), or covalent bonds where it oxidizes other halogens (ClF, ClF3, ClF5). It will react explosively with many elements and compounds such as hydrogen and water. Elemental fluorine is slightly basic, which means that when it reacts with water it forms OH−.
2F2+2H2O→O2+4HF
When combined with hydrogen, fluorine forms hydrofluoric acid (HF), which is a weak acid. This acid is very dangerous and when dissociated can cause severe damage to the body because while it may not be painful initially, it passes through tissues quickly and can cause deep burns that interfere with nerve function.
HF+H2O→H3O++F−
There are also some organic compounds made of fluorine, ranging from nontoxic to highly toxic. Fluorine forms covalent bonds with carbon, which sometimes form into stable aromatic rings. When carbon reacts with fluorine, the reaction is complex and forms a mixture of CF4, C2F6, and C5F12.
C(s)+F2(g)→CF4(g)+C2F6+C5F12
Fluorine reacts with oxygen to form OF2 because fluorine is more electronegative than oxygen. The reaction goes:
2F2+O2→2OF2
Fluorine is so electronegative that sometimes it will even form molecules with noble gases like Xenon, such as the molecule xenon difluoride, XeF2.
Xe+F2→XeF2
Fluorine also forms strong ionic compounds with metals. Some common ionic reactions of fluorine are:
F2+2NaOH→O2+2NaF+H2
4F2+HCl+H2O→3HF+OF2+ClF3
F2+2HNO3→2NO3F+H2
Applications of Fluorine
Compounds of fluorine are present in fluoridated toothpaste and in many municipal water systems, where they help to prevent tooth decay. And, of course, fluorocarbons such as Teflon have made a major impact on life in the 20th century. There are many applications of fluorine:
- Rocket fuels
- Polymer and plastics production
- Teflon and tefzel production
- When combined with oxygen, used as a refrigerator cooler
- Hydrofluoric acid used for glass etching
- Purify public water supplies
- Uranium production
- Air conditioning
Sources
Fluorine can either be found in nature or produced in a lab. To make it in a lab, compounds like potassium fluoride are put through electrolysis with hydrofluoric acid to create pure Fluorine and other compounds. It can be carried out with a variety of compounds, usually ionic ones involving fluorine and a metal. Fluorine can also be found in nature in various minerals and compounds. The two main compounds it can be found in are Fluorspar (CaF2) and Cryolite (Na3AlF6).
References
- Newth, G. S. Inorganic Chemistry. Longmans, Green, and Co.:New York, 1903.
- Latimer, Wendell M., Hildebrand, Joel H. Reference Book of Inorganic Chemistry. The Macmillan Company: New York, 1938.
Problems
(Highlight to view answers)
1. Q. What is the electron configuration of fluorine? of F-?
A. 1s22s22p5
1s22s22p6
2. Q. Is fluorine usually oxidized or reduced? explain.
A. Fluorine is usually reduced because it accepts an electron from other elements since it is so electronegative.
3. Q. What are some common uses of fluorine?
A. Toothpaste, plastics, rocket fuels, glass etching, etc.
4. Q. Does fluorine form compounds with nonmetals? if so, give two examples, one of them being of an oxide.
A. OF2, ClF
5. Q. What group is fluorine in? (include name of group and number)
A. 17, Halogens
Contributors and Attributions
- Rachel Feldman (University of California, Davis)
Stephen R. Marsden

