Covalent Bonds
- Page ID
- 1984
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Covalent bonding occurs when pairs of electrons are shared by atoms. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full electron shell. By sharing their outer most (valence) electrons, atoms can fill up their outer electron shell and gain stability. Nonmetals will readily form covalent bonds with other nonmetals in order to obtain stability, and can form anywhere between one to three covalent bonds with other nonmetals depending on how many valence electrons they posses. Although it is said that atoms share electrons when they form covalent bonds, they do not usually share the electrons equally.
Introduction
Only when two atoms of the same element form a covalent bond are the shared electrons actually shared equally between the atoms. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher electronegativity resulting in a polar covalent bond. When compared to ionic compounds, covalent compounds usually have a lower melting and boiling point, and have less of a tendency to dissolve in water. Covalent compounds can be in a gas, liquid, or solid state and do not conduct electricity or heat well. The types of covalent bonds can be distinguished by looking at the Lewis dot structure of the molecule. For each molecule, there are different names for pairs of electrons, depending if it is shared or not. A pair of electrons that is shared between two atoms is called a bond pair. A pair of electrons that is not shared between two atoms is called a lone pair.
Octet Rule
The Octet Rule requires all atoms in a molecule to have 8 valence electrons--either by sharing, losing or gaining electrons--to become stable. For Covalent bonds, atoms tend to share their electrons with each other to satisfy the Octet Rule. It requires 8 electrons because that is the amount of electrons needed to fill a s- and p- orbital (electron configuration); also known as a noble gas configuration. Each atom wants to become as stable as the noble gases that have their outer valence shell filled because noble gases have a charge of 0. Although it is important to remember the "magic number", 8, note that there are many Octet rule exceptions.
Example: As you can see from the picture below, Phosphorus has only 5 electrons in its outer shell (bolded in red). Argon has a total of 8 electrons (bolded in red), which satisfies the Octet Rule. Phosphorus needs to gain 3 electrons to fulfill the Octet Rule. It wants to be like Argon who has a full outer valence shell.
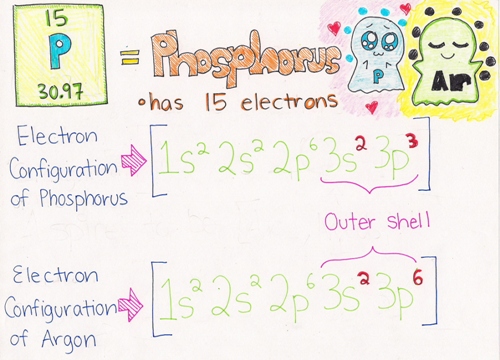
More examples can be found here.
Single Bonds
A single bond is when two electrons--one pair of electrons--are shared between two atoms. It is depicted by a single line between the two atoms. Although this form of bond is weaker and has a smaller density than a double bond and a triple bond, it is the most stable because it has a lower level of reactivity meaning less vulnerability in losing electrons to atoms that want to steal electrons.
Below is a Lewis dot structure of Hydrogen Chloride demonstrating a single bond. As we can see from the picture below, Hydrogen Chloride has 1 Hydrogen atom and 1 Chlorine atom. Hydrogen has only 1 valence electron whereas Chlorine has 7 valence electrons. To satisfy the Octet Rule, each atom gives out 1 electron to share with each other; thus making a single bond.

Double Bonds
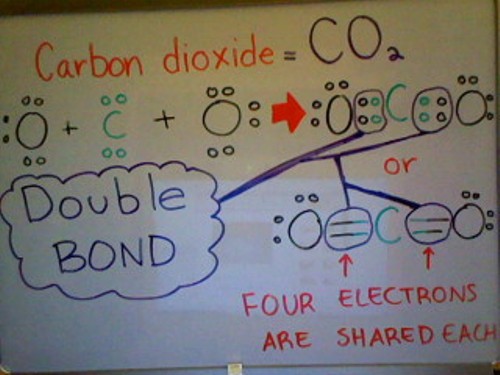
A Double bond is when two atoms share two pairs of electrons with each other. It is depicted by two horizontal lines between two atoms in a molecule. This type of bond is much stronger than a single bond, but less stable; this is due to its greater amount of reactivity compared to a single bond.
Below is a Lewis dot structure of Carbon dioxide demonstrating a double bond. As you can see from the picture below, Carbon dioxide has a total of 1 Carbon atom and 2 Oxygen atoms. Each Oxygen atom has 6 valence electrons whereas the Carbon atom only has 4 valence electrons. To satisfy the Octet Rule, Carbon needs 4 more valence electrons. Since each Oxygen atom has 3 lone pairs of electrons, they can each share 1 pair of electrons with Carbon; as a result, filling Carbon's outer valence shell (Satisfying the Octet Rule).
Triple Bond
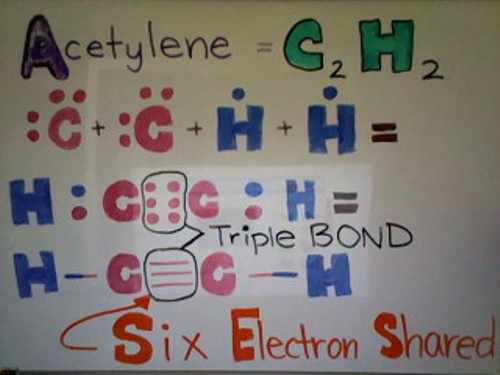
A Triple bond is when three pairs of electrons are shared between two atoms in a molecule. It is the least stable out of the three general types of covalent bonds. It is very vulnerable to electron thieves!
Below is a Lewis dot structure of Acetylene demonstrating a triple bond. As you can see from the picture below, Acetylene has a total of 2 Carbon atoms and 2 Hydrogen atoms. Each Hydrogen atom has 1 valence electron whereas each Carbon atom has 4 valence electrons. Each Carbon needs 4 more electrons and each Hydrogen needs 1 more electron. Hydrogen shares its only electron with Carbon to get a full valence shell. Now Carbon has 5 electrons. Because each Carbon atom has 5 electrons--1 single bond and 3 unpaired electrons--the two Carbons can share their unpaired electrons, forming a triple bond. Now all the atoms are happy with their full outer valence shell.
Polar Covalent Bond
A Polar Covalent Bond is created when the shared electrons between atoms are not equally shared. This occurs when one atom has a higher electronegativity than the atom it is sharing with. The atom with the higher electronegativity will have a stronger pull for electrons (Similiar to a Tug-O-War game, whoever is stronger usually wins). As a result, the shared electrons will be closer to the atom with the higher electronegativity, making it unequally shared. A polar covalent bond will result in the molecule having a slightly positive side (the side containing the atom with a lower electronegativity) and a slightly negative side (containing the atom with the higher electronegativity) because the shared electrons will be displaced toward the atom with the higher electronegativity. As a result of polar covalent bonds, the covalent compound that forms will have an electrostatic potential. This potential will make the resulting molecule slightly polar, allowing it to form weak bonds with other polar molecules. One example of molecules forming weak bonds with each other as a result of an unbalanced electrostatic potential is hydrogen bonding, where a hydrogen atom will interact with an electronegative hydrogen, fluorine, or oxygen atom from another molecule or chemical group.
Example: Water, Sulfide, Ozone, etc.
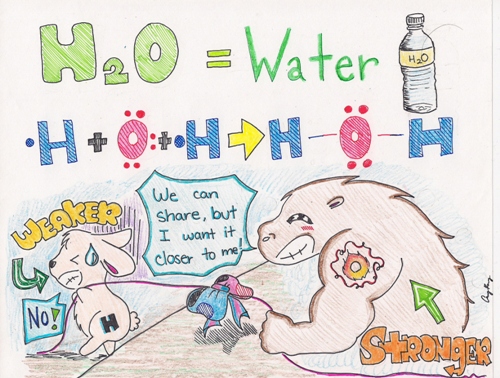
As you can see from the picture above, Oxygen is the big buff creature with the tattoo of "O" on its arm. The little bunny represents a Hydrogen atom. The blue and red bow tied in the middle of the rope, pulled by the two creatures represents--the shared pair of electrons--a single bond. Because the Hydrogen atom is weaker, the shared pair of electrons will be pulled closer to the Oxygen atom.
Nonpolar Covalent Bond
A Nonpolar Covalent Bond is created when atoms share their electrons equally. This usually occurs when two atoms have similar or the same electron affinity. The closer the values of their electron affinity, the stronger the attraction. This occurs in gas molecules; also known as diatomic elements. Nonpolar covalent bonds have a similar concept as polar covalent bonds; the atom with the higher electronegativity will draw away the electron from the weaker one. Since this statement is true--if we apply this to our diatomic molecules--all the atoms will have the same electronegativity since they are the same kind of element; thus, the electronegativities will cancel each other out and will have a charge of 0 (i.e., a nonpolar covalent bond).
Examples of gas molecules that have a nonpolar covalent bond: Hydrogen gas atom, Nitrogen gas atoms, etc.
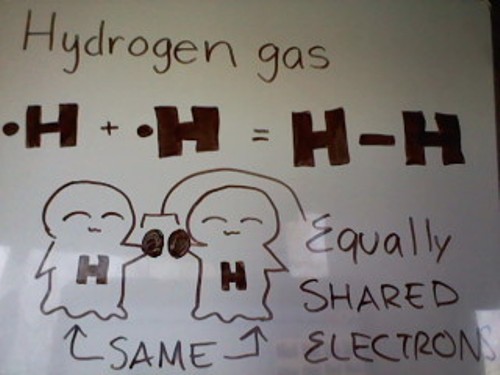
As you can see from the picture above, Hydrogen gas has a total of 2 Hydrogen atoms. Each Hydrogen atom has 1 valence electron. Since Hydrogen can only fit a max of 2 valence electrons in its orbital, each Hydrogen atom only needs 1 electron. Each atom has 1 valence electron, so they can just share, giving each atom two electrons each.
References
- Petrucci, Ralph H., Harwood, William S., Herring, F. G., and Madura Jeffrey D. "General Chemistry: Principles & Modern Applications." 9th Ed. New Jersey: Pearson Education, Inc., 2007. Print.
- Vaczek, Louis. "The Enjoyment of Chemistry." New York: Viking Press, 1968.
- Pickering, H. S. "The Covalent Bond." London: Wykeham Publications Ltd., 1977.
- Kotz, Treichel, Townsend. "Chemistry and Chemical Reactivity: OWL E-Book Edition." 7th Ed. Ohio: Cengage Learning, 2008.
- Lagowski, J. J. "The Chemical Bond." Boston: Houghton Mifflin Company, 1966.
- Bacskay, George G.; Reimers, Jeffrey R.; Nordholm, Sture. "The Mechanism of Covalent Bonding." J. Chem. Educ. 1997 74 1494.
- Reimers, Jeffrey R.; Bacskay, George G.; Nordholm, Sture. "The Basics of Covalent Bonding." J. Chem. Educ. 1997 74 1503.
Outside Links
- Covalent Bond - Wikipedia: en.Wikipedia.org/wiki/Covalent_bond
- Electron Sharing and Covalent Bonds - http://www.chem.ox.ac.uk/vrchemistry...nds/intro1.htm
- Bond Stability - Newton BBS: www.newton.dep.anl.gov/askasc.../chem03155.htm
- Covalent Radii - Wikipedia: http://en.Wikipedia.org/wiki/Covalent_radius
Problems
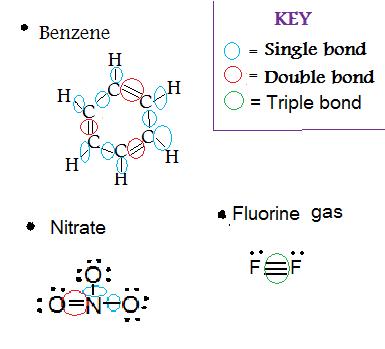
1. Determine the type(s) of bond(s) in
- Benzene (C6H6)
- NO3 (Nitrate)
- F2(Fluorine gas)
Solution:
2. Write the electron configuration and determine how many electrons are needed to achieve the nearest noble-gas configuration for the following:
- Arsenic (As)
- Silicon (Si)
- Tellurium (Te)
Solution:

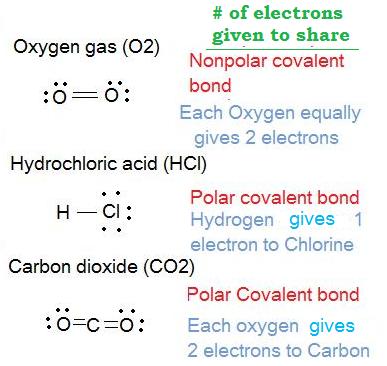
3. Determine which molecules are polar and which molecules are nonpolar for the following:
- Oxygen gas (O2)
- Hydrochloric acid (HCl)
- Carbon dioxide (CO2)
Solution:
4. Which of the following statements are true? (There can be more than one true statement.)
- A covalent bond is the same as a ionic bond.
- The Octet rule only applys to molecules with covalent bonds.
- A molecule is polar if the shared electrons are equally shared.
- A molecule is nonpolar if the shared electrons are are equally shared.
- Methane gas (CH4) has a nonpolar covalent bond because it is a gas.
Solution: Only d) is true.
5. Match each atom or molecule with its corresponding letter(s):
- Nitrogen gas
- Argon
- Carbon monoxide
- Hydrogen gas
a) Nonpolar covalent bond
b) Polar covalent bond
c) Follows the Octet Rule
d) Noble gas
e) Two lone pairs
f) Single bond
Solution:
- Nitrogen gas: a), c), e)
- Argon: c), d)
- Carbon monoxide: b), c), e)
- Hydrogen gas: c), f)
Contributors and Attributions
- Camy Fung (UCD), Nima Mirzaee (UCD)

