XANES: Application
- Page ID
- 1868
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)XANES, short for X-ray Absorption Near-Edge Structure, is a subset of X-ray Absorption Spectroscopy. The absorption edge corresponding to the liberation of a core electron from an element will exhibit several identifiable features which change depending on the chemical environment of the element being probed. The study and modelling of the characteristics of near-edge features helps answer questions about the oxidation state, coordination, and spin state of the probed element.
Introduction
X-ray Absorption Near-Edge Structure (XANES), though less-developed or practiced than Extended X-ray Absorption Fine Structure (EXAFS), may provide valuable information about the oxidation state, coordination environment, and bonding characteristics of specific elements in a sample. The less common term Near-Edge X-ray Absorption Fine Structure (NEXAFS) is used generally in the context of solid-state studies and is synonymous with XANES. The technique in practice requires a mix of qualitative and quantitative analysis to interpret the data and draw conclusions.
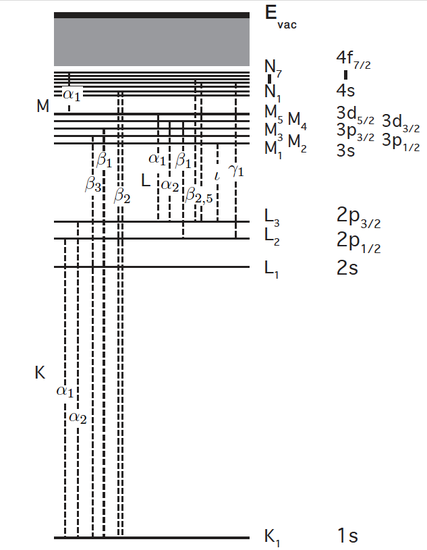
The mechanisms and terminology in XANES are a subset of X-ray Absorption spectroscopy (XAS) and will only be summarized here. The absorption edges of a material are the element-specific sudden increase in the absorption coefficient due to the promotion of a core-level electron to unoccupied orbitals or unbound state. The core electron may be a member of the s, p, d, or higher-order orbitals, provided it is not a valence (outer shell) electron. "XAS" refers to the nature of the electron that is excited rather than the energy range of the exciting photon. Edges resulting from the excitation of an n=1 (1s) electron are termed "K-edges", n=2 are "L-edges", n=3 "M-edges", and so on. The figure below labels these shells and corresponding allowed bound-state absorption levels that are seen as pre- and on-edge features in XANES. Dotted lines correspond to observed emission lines. When an orbital at the top of a dotted line is not full, the dotted line also indicates an absorption from a lower shell (a bound-state transition). For example, the K-edge of Fe has an on-edge bound-state feature corresponding to 1s -> 4p absorption, which would be the dotted line in the figure between K1 and N2,3. These follow selection rules ∆L = +/- 1.
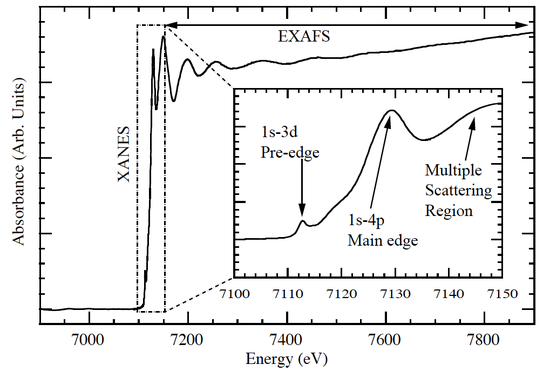
XANES is the study of the features immediately before and after the edge, within approximately 1% on either side of the main edge energy. Features include the edge position (a primary indicator of oxidation state), presence/shape of small features just before the main edge ("pre-edge" features), and intensity, number, position and shape of peaks at the top of the main edge. The Fe K-edge shown below from Carpenter (2010) labels some of these features. The pre-edge feature is weak as it involves a forbidden transition (which is partially allowed due to mixing of ligand p-character), while the first line on the main edge is due to the allowed 1s to 4p bound-state transition. In some compounds this transition is much stronger than the rest of the edge (see Sulfur K-edge in next figure) and was historically called the "white line" due to its saturated appearance on the photographic film used to record the spectrum.
The power of XANES lies in the sensitivity of edge features to the chemical environment. The sensitivity varies among elements, from just-detectable to pronounced. George et. al provide an excellent illustration of the range of the sulfur K edge among various organic compounds. [3]
Instrumentation
Unlike visible, infrared, or microwave spectroscopy, no single off-the-shelf commercial instrument exists for XAS. The equipment must be assembled from multiple components to suit the needs of the experiment and may involve a significant amount of custom engineering. Laboratory X-ray sources were used before the advent of synchrotron radiation sources, but today they are rarely considered. The typical XAS experiment at a synchrotron may be broken down into two general parts:
- The "beam line": the set of components (bend magnet or undulator/wiggler, mirrors, monochromator(s), slits, diagnostics) that produce and deliver a controllable high-intensity monochromatic X-ray beam.
- The "end station": the set of instruments specific to the type of measurement, including sample handling (gas cells, fluid cells, cryostats, positioning stages), detectors, diagnostics and support systems, and radiation protection enclosure.
XANES measurements use essentially the same equipment and setup as EXAFS, though as the energy range covered is much smaller the emphasis may be on high-resolution over wide-range abilities. A typical beam line schematic is shown below.

Measurement Methods
| Transmission | Electron Yield | Fluorescence Yield | |
|---|---|---|---|
| Sample Thickness | Thin | Thick/Any | Thick/Any |
| Background | High | Moderate | Low |
| Sensitivity | Bulk | Surface | Bulk |
| Sample Concentration | High | High | Low |
Transmission
As the name implies, this method involves passing X-rays through the sample and comparing the incident to the transmitted intensities. The sample thickness must be considered before measurement, as absorption coefficients vary greatly across the X-ray energy range and among materials. For too-thick samples the beam may be totally attenuated at the edge, while too-thin results in poor signal-to-noise ratio. For ultra-soft measurements the sample may need to be less than 1 micron thick, while high-Z K-edges may need many centimeters of sample. Reference databases such as the LBNL Center for X-ray Optics database give attenuation data and calculation tools to help estimate the proper thickness, which will depend on the concentration of the measured species and the attenuation of other species present.
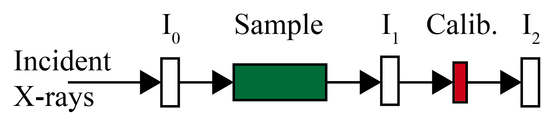
Measurement of the intensity may be made with gas ionization chambers, photodiodes, PN-junctions, metal grids, or from scattering off optics or windows. The incident beam (I0) must be only partially sampled, typically by an ion chamber or grid, while the transmitted beam (I1) may be blocked and absorbed completely. A helpful calibration technique is to place a reference standard and detector after the I1 detector, so that the beam path looks as in the following diagram so the reference spectrum is measured simultaneous with the sample.
Transmission measurements may be performed on any type of sample (gas, liquid, solid) provided the thickness and density is controllable. For dilute measurements the signal-to-noise ratio is typically poor.
Electron Yield
Absorption of X-rays results in emission of electrons from the sample proportional to the absorption coefficient, from both photoelectrons and Auger electrons. Photoelectrons are those that are ejected from core orbitals and will have a kinetic energy that is the difference of the X-ray energy and their binding energy. Auger electrons are emitted as part of the relaxation process as a higher-orbital electron fills the hole left behind by the photoelectron. The Auger electron energy is characteristic of the element and core level being occupied and is analogous to a fluorescence photon. Collecting all produced electrons is known as Total Electron Yield (TEY) and is measured with electron multipliers electrical current through a lead (in vacuum), or via gas ionization collected by a grid close to the sample surface (non-vacuum). Alternately, an electron energy analyzer may be used to discriminate between photo and Auger electrons, which helps reject background from other species at the expense of throughput. This method is Partial Electron Yield (PEY). This method is only sensitive to the first ~10s of nm of the sample surface due to electron scattering, and only works for solid samples. This may be an advantage for thin films or monolayers on a substrate where other methods would suffer high background issues. Auger electrons and fluorescence are competing processes and the ratio between the two changes depending on the element. Auger yield predominates for light elements like C and N, but gradually decreases in favor of fluorescence as Z increases. The crossover where fluorescence is greater than Auger yield is Z=30.[27]
Fluorescence Yield
As with electron yield, X-ray fluorescence is proportional to the absorption coefficient as valence electrons emit photons to fill the core holes left by absorption. As with electron yield, fluorescence yield may be measured in total or partial modes, the later requiring an energy-discriminating detector. For partial-yield, solid-state detectors such as Germanium and drifted Silicon detectors are often used due to their high efficiency and moderate energy resolution (enough to separate emission from different species). Dispersive spectrometers with gratings or Bragg crystals may also be used when higher resolution is needed to separate species emissions that are close-together. The detector is typically oriented perpendicular to the incident beam polarization to suppress the elastic scattering peak and improve signal-to-noise. Total yield may be measured with any X-ray-sensitive detector not immediately in the incident or transmitted beam.
Fluorescence yield is especially well-suited for dilute samples due to its selectivity and bulk-sensitivity. However, when used for concentrated samples, a phenomenon called "self-absorption" can lower the apparent absorption coefficient at high levels of absorption. This is partially due to non-negligible reduction in the penetration depth as the absorptivity increases (directly impacting the coefficient due to Beer's law), and re-absorption of the fluorescence photons by the same species before the photons can leave the sample.
Hard X-ray Instrumentation
Where "Hard" X-rays begin varies widely, typically between 2 and 10 keV photon energy. They are the X-rays that are high enough in energy to penetrate significant distances through materials. This penetrating capacity is a blessing and a curse: many materials may be used as windows, the sample can be in a variety of environments, and measurements are less sensitive to thickness tolerances; however, optics must take this penetration into account, and complete shielding is needed to protect the user from radiation. The K-edges of first-row transition metals and L-edges of rare earth elements fall in this range. Solid samples may be contained in a metal or plastic holder with low-Z material windows. Liquids and gases may require custom sample alignment which is usually automated with motorized stages. The endstation is shielded from the user by a protective hutch. Radiation-sensitive samples may be cooled to low temperatures (liquid Nitrogen or Helium temperature) in a cryostat modified with X-ray windows. The X-ray beam often passes from the high-vacuum beamline through a Beryllium window and travels moderate distances through air or an exchange gas such as Helium before impinging the sample. Transmission and fluorescence yields are the primary measurement methods.
Soft X-ray Instrumentation
"Soft" X-rays are those that are readily absorbed by most materials and air with very short attenuation lengths, roughly in the range of ~100eV to 2-5 keV. The entire experiment (sample, detector, diagnostics) must be contained in vacuum up to Ultra-High Vacuum (UHV). Window materials are few and must be thin. Sample containment must take into account in the vacuum and window materials. Most often, the sample is inserted directly into vacuum as a solid (as a powder, crystal or amorphous solid), or dried a substrate. Gas cells are also used for appropriate samples and for reaction/catalysis experiments. Electron and fluorescence yields predominate in Soft X-rays.
Data handling and Analysis
The treatment of data depends on the compound complexity and nature of the problem solved. Very complicated molecules which are difficult to simulate with software may be compared to simpler model compounds to determine coordination electronic structure.
Recent advances in theoretical calculations and software allow general users to fit the spectrum based on ionization state, coordination group, and various molecular parameters such as crystal field splitting and degree of orbital hybridization. The post-edge region dominated by multiple-scattering may provide structural information directly similar to EXAFS, though by a different approach. While simple-scattering in EXAFS is derived from the Fourier transform of the post-edge oscillations, multiple-scattering involves fully modeling the quantum-mechanical spherical wave scattering from local neighbors via Green's functions.[16]
Other software packages utilizing Density Functional Theory (DFT) or Charge-Transfer Multiplet Theory (CTM) are used to derive the manifold of atomic or molecular orbital energies from bound-state transitions.[11]
Figure 1 from Metzler et. al[9] illustrates the assignment of components from simulation which may be correlated with the observed spectrum. The assignments of the features by Metzler et al. are as follows: "peak 1 at 285 eV, corresponds to the C ls → ∏* transition in C=C double bonds; peak 2 at 287 eV is the C-H C ls → ∑*, mostly due to PLL side chains; peaks 3 and 4 at ~288 eV are both associated with C=0 C ls → ∏*; peak 5 at ~290 eV, corresponds to the C ls → ∏* transition in C=0 double bonds in carbonates." They include a dotted feature for the absorption edge due to ionization (core electron -> unbound continuum states).
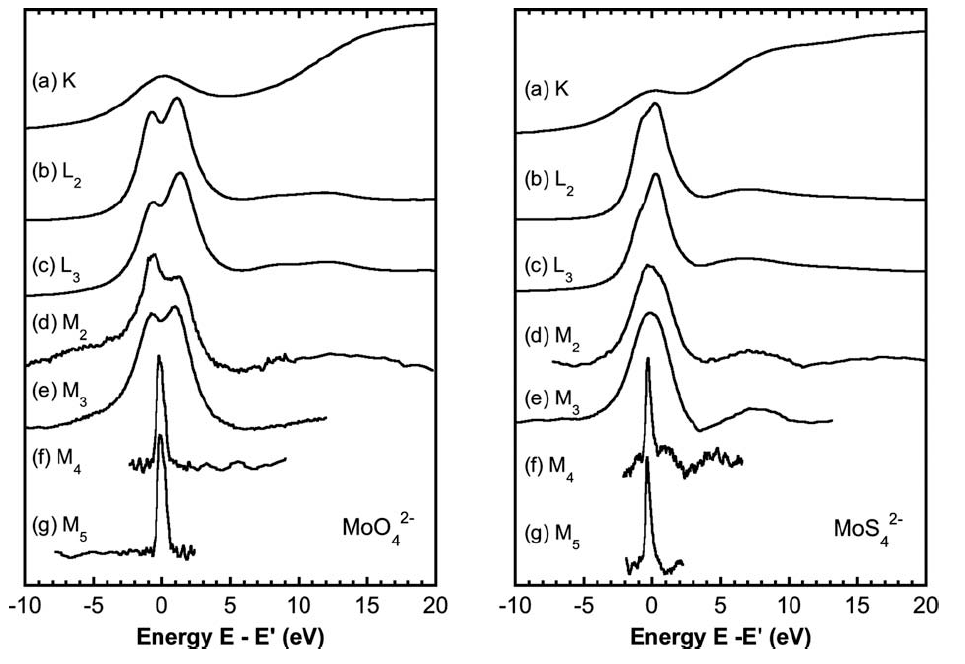
Investigation of multiple edges of the same element may also offer insight, as different features are emphasized at different edges. The lower-energy edges are usually more highly-resolved than the K-edge due to longer core-hole lifetimes (by the Heisenberg uncertainty principle, longer-lived states are less broad in energy) and allow the ionization state to be determined more accurately. A comparison of the K, L, and M-edges of Molybdenum is shown below in which the analogous feature (bound state transitions to d orbitals) is lined up at 0 eV in each spectrum.
Applications
Biomolecules
The understanding of large molecules such as proteins can benefit greatly from XANES. The active-site clusters often contain one or several metal ions embedded in bulk proteins. The presence of large amounts of organic backbone may interfere with UV-Vis spectroscopy of the active site, while XANES, being element-specific, allows single atoms to be measured in even large proteins. Preparation of the enzyme in resting and active states before the measurement and careful handling during the measurement may elucidate the oxidation and coordination state. Depending on the substrate, the bound-state may be measured from both the metal and ligand XAS.
A literature example of the sort of information which can be derived from XANES is the paper by Ralston et. al [20] to determine the spin state of Ni in the active site of CO-Dehydrogenase (CODH). Through a careful study of multiple model compounds of known oxidation states and spin configurations, from Ni(I), to low- and high-spin Ni(III) up to Ni(IV), a relationship is derived between the position of the L3 edge and the ratio of the integrals of the L3 and L2 edges. A large ratio between the two edges indicates a high-spin complex while lower ratio indicates low-spin, with the turning point occurring at a ratio of 0.71 L3:L2 intensity. This scheme is applied to CODH prepared as a film in its resting state, CO-bound state, and dithionate-reduced state to determine the Ni oxidation and spin states.
Solid State physics
Edge features corresponding to bound-state transitions will have the symmetries of the immediate coordination environment (that surrounding the probed element only). For solid state samples in a single-crystal form, this may be combined with the fact that synchrotron radiation is typically linearly polarized in the plane of the storage ring to probe specific orbitals of the element. Electronic structure and band gap measurements are therefore possible. [21,25]
Materials science
Materials scientists often care about the behavior of materials under extreme conditions. High-pressure diamond cells combined with powerful lasers allow the probing of materials in the regions of 5000K and 100s of GPa.[24] Small X-ray beam spot sizes on the order of microns allow so-called "Micro-XANES" measurements to probe changes along pressure gradients of materials in these cells. Temperature-dependent XANES measurements can capture phase transitions in materials and give insight into structural changes.[22]
Development of novel materials such as high-Tc superconductors and advanced scintillators can also benefit from XANES.[23] The low-concentration of dopants in scintillators is perfectly suited to fluorescence-yield XAS to determine concentration and ionization state. The plot below depicts the M4 and M5 edges of europium doped at 1% measured by partial fluorescence yield.

Catalysis
Specially-designed cells combined with XANES may provide insight into reaction dynamics and changes in electronic configuration and oxidation state. Performing the reaction under different temperatures and conditions gives insight into dynamics. With the development of fast, continuous-scan beamlines with the capability to scan an entire edge in less than a minute, time-resolved studies are now possible as well. Leoferti et. al followed the Cu oxidation state during oxychlorination of ethylene catalyzed by copper chloride,[26] which was built-upon later by Lamberti et. al with time-resolved XANES with 30-second resolution.[13] Further experiments are possible with the development of reaction cells which allow multiple simultaneous measurements, such as XANES, UV-Vis and electro-chemistry.[14]
Surface Science
The shallow penetration depth of soft X-rays combined with the probing depth of electron yield measurement allows for very sensitive studies on films as thin as a monolayer.[15] When deposited on a single-crystal surface in controlled conditions, the monolayer species can be made to bond with a particular orientation to that surface allowing for polarization-dependent studies. As the X-rays emitted by bend magnets and standard wigglers or undulators is linearly polarized in the plane of the ring, changing the orientation of the crystal to the incident beam can suppress or enhance features in the absorption edge. The monolayer bonding of the sample to substrate may also alter the characters of the edge independent of the polarization[17] which opens up further possibilities for understanding the system.
Sample Diagnostics
Experiments which benefit from synchrotron radiation, such as X-ray crystallography, Scanning Transmission X-ray Microstopy (STXM), and X-ray Photo-emission Electron Microscopy (XPEEM), typically need large doses of radiation to be effective. For radiation-sensitive samples the dose may be an important consideration as to the validity of the data: for crystallography, the high-energy electrons liberated from atoms after ionization may distort the very structure one is trying to measure, while STXM and PEEM may damage or destroy the large bio-structures often probed with these methods. XANES, combined with an appropriate model for the absorption of radiation and its affects on the edge features, may be used to both set limits and parameters for the experiment[18,19] and monitor the sample condition as the experiment progresses.
References
- Frank de Groot and Akio Kotani, "Core Level Spectroscopy of Solids", CRC Press, Boca Raton, FL (2008).
- Joachim Sthöhr, "NEXAFS Spectroscopy", Second Printing, Springer-Verlag, Heidelberg, Germany (2003).
- Graham N. George, Martin L. Gorbaty, "Sulfur K-edge x-ray absorption spectroscopy of petroleum asphaltenes and model compounds"; J. Am. Chem. Soc. 111 (9), pp 3182–3186 (1988).
- C. R. Natoli, D. K. Misemer, S. Doniach, and F. W. Kutzler, "First-principles calculation of x-ray absorption-edge structure in molecular clusters"; Phys. Rev. A 22, 1104–1108 (1980).
- Owen B. Drury, "Development of High Resolution X-Ray Spectrometers for the Investigation of Bioinorganic Chemistry in Metalloproteins"; Ph.D. Thesis, University of California, Davis (2007).
- H. Oyanagi, Z. H. Sun, Y. Jiang, M. Uehara, H. Nakamura, K. Yamashita, L. Zhang, C. Lee, A. Fukanoa, and H. Maeda, "In situ XAFS experiments using a microfluidic cell: application to initial growth of CdSe nanocrystals"; J. Synchrotron Radiaion 18, 272–279 (2011).
- Simon J. George, Owen B. Drury, Juxia Fu, Stephan Friedrich, Christian J. Doonan, Graham N. George, Jonathan M. White, Charles G. Young, Stephen P. Cramer, "Molybdenum X-ray absorption edges from 200 to 20,000 eV: The benefits of soft X-ray spectroscopy for chemical speciation"; J. Inog. Biochem. 103, 157–167 (2009).
- Matthew H. Carpenter, "Helium Atmosphere Chamber for Soft X-ray Spectroscopy of Biomolecules", MS Thesis, University of California, Davis (2010).
- Rebecca A. Metzler, Ronke M. Olabisi, Mike Abrecht, Daniel Ariosa, Christopher J. Johnson, Benjamin Gilbert, Bradley H. Frazer, Susan N. Coppersmith, and P.U.P.A Gilbert, "XANES in Nanobiology"; Proceedings of X-ray Absorption Fine Structure—XAFSU 13, American Institute of Physics, 51 (2007).
- S. Della Longa, A. Arcovito, M. Girasole, J. L. Hazemann, and M. Benfatto, "Quantitative Analysis of X-Ray Absorption Near Edge Structure Data by a Full Multiple Scattering Procedure: The Fe-CO Geometry in Photolyzed Carbonmonoxy-Myoglobin Single Crystal"; Phys. Rev. Lett. 87, 15, 155501 (2001).
- E. Stavitski and F.M.F. de Groot, "The CTM4XAS program for EELS and XAS spectral shape analysis of transition metal L edges"; Micron 41, 687 (2010).
- Takashi Fujikawa, "Basic Features of the Shrort-Range-Order Multiple Scattering XANES Theory"; J. Phys. Soc. Japan 62, 6, pp. 2115 (1993).
- Carlo Lamberti, Carmelo Prestipino, Francesca Bonino, Luciana Capello, Silvia Bordiga, Giuseppe Spoto, Adriano Zecchina, Sofia Diaz Moreno, Barbara Cremaschi, Marco Garilli, Andrea Marsella, Diego Carmello, Sandro Vidotto, and Giuseppe Leofanti, "The Chemistry of the Oxychlorination Catalyst: an In Situ, Time-Resolved XANES Study"; Angew. Chem. Int. Ed. 41, No. 13, pp 2341 (2002).
- Walkiria S. Schlindwein, Aristea Kavvada, Roger G. Linford, Roger J. Latham and J. Günter Grossmann, "Combined XAS/SAXS/Electrochemical studies on the conformation of poly(vinylferrocene) under redox conditions"; Ionics 8, 1-2, 85-91 (2002).
- A. Biancoli, "Surface X-ray absorption spectroscopy: Surface EXAFS and surface XANES"; Applications of Surface Science 6, 3-4, 392-418 (1980).
- M. Benfatto, C. R. Natoli, A. Bianconi, J. Garcia, A. Marcelli, M. Fanfoni, and I. Davoli, "Multiple-scattering regime and higher-order correlations in x-ray-absorption spectra of liquid solutions"; Phys. Rev. B 34, 5774–5781 (1986).
- E. E. Doomes, P. N. Floriano, R. W. Tittsworth, R. L. McCarley, and E. D. Poliakoff, "Anomalous XANES Spectra of Octadecanethiol Adsorbed on Ag(111)"; Phys. Chem. B 107 (37), 10193–10197 (2003).
- J.Wang, C. Morin, L. Li, A.P. Hitchcock, A. Scholl, A. Doran, "Radiation damage in soft X-ray microscopy"; Journal of Electron Spectroscopy and Related Phenomena 170, 25–36 (2009).
- James W. Murray, Enrique Rudiño-Piñera, Robin Leslie Owen, Martin Grininger, Raimond B. G. Ravelli, and Elspeth F. Garmana, "Parameters affecting the X-ray dose absorbed by macromolecular crystals"; J. Synchrotron Rad. 12, 268-275 (2005).
- C. Y. Ralston, Hongxin Wang, S. W. Ragsdale, M. Kumar, N. J. Spangler, P. W. Ludden, W. Gu, R. M. Jones, D. S. Patil, and S. P. Cramer, "Characterization of Heterogeneous Nickel Sites in CO Dehydrogenases from Clostridium thermoaceticum and Rhodospirillum rubrum by Nickel L-Edge X-ray Spectroscopy"; J. Am. Chem. Soc. 122, 10553-10560 (2000).
- O. Seifarth, J. Dabrowski, and P. Zaumseil, S. Müller and D. Schmeißer, H.-J. Müssig, and T. Schroeder."On the band gaps and electronic structure of thin single crystalline praseodymium oxide layers on Si(111)"; J. Vac. Sci. Technol. B 27, 271 (2009).
- B. Ravel and E.A. Stern, "Temperature and Polarization Dependent XANES Measurements on Single Crystal PbTiO3"; J. Phys IV France 7, 1223 (1997).
- Stephan Friedrich, Owen B. Drury, Shaopang Yuan, Piotr Szupryczynski, Merry A. Spurrier, and Charles L. Melcher, "A 36-Pixel Tunnel Junction Soft X-Ray Spectrometer for Scintillator Material Science"; IEEE Transactions on Applied Superconductivity 17, 2, 351 (2007).
- Giuliana Aquilanti, Sakura Pascarelli, Olivier Mathon, Manuel Muñoz, Olga Naryginac, and Leonid Dubrovinsky, "Development of micro-XANES mapping in the
diamond anvil cell"; J. Synchrotron Rad. 16, 376–379 (2009). - V. L. Mazalova and A. V. Soldatov, "Geomtrical and Electronic Structure of Small Copper Nanoclusters: XANES and DFT Analysis"; Journal of Structural Chemistry 49, Supplement, S107-S115 (2008).
- G. Leofanti, A. Marsella, B. Cremaschi, M. Garilli, A. Zecchina, G. Spoto, S. Bordiga, P. Fisicaro, C. Prestipino, F. Villain, and C. Lamberti, "Alumina-Supported Copper Chloride: 4. Effect of Exposure to O2 and HCl"; Journal of Catalysis 205, 375–381 (2002).
- Krause, M.O., and Oliver J.H., "Natural Widths of Atomic K and L Levels, Ka XRay Lines and Several KLL Auger Lines"; Journal of Chemical and Physical
Reference Data 8(2), 329-337 (1979).
Simulation Software
- CTM4XAS (ionization and coordination chemistry)
- FEFF (multiple scattering)
- FitIt graphical front-end for suite of fitting software.
Problems
- As the oxidation state of an atom increases, which direction does the absorption edge shift (higher or lower in energy)?
- Name 2 characteristcs which may be determined by XANES.
- What measurement method is most appropriate for measuring the Cu K-edge of the protein Stellacyanin? What would be most appropriate for the Ni L-edge of NiO?
- List the energies of the K- and L-edges of Si, Fe, and Zn, and the K-edges of C, N, P and S.
- Why are pre-edge features corresponding to forbidden transitions sometimes observed on metal K-edges?
Solutions
- The edge shifts higher in energy. The lesser amount of shielding of the nucleus by surrounding electrons increases its effective charge Zeff; the more tightly-bound electrons require more energy to liberate.
- Possible answers: oxidation state, valence (number of ligands), coordination geometry, near-neighbor distances (through multiple-scattering).
- Stellacyanin is a 20 kDa protein with 1 Cu, putting it in the "dilute" regime; it should be measured by Partial Fluorescence Yield. NiO is stochiometrically 1/2 Ni; due to the concentration, the high fluorescence-yield of Ni, and the fact that the Ni L-edge lies in the soft X-ray region means it should be measured by Total or Partial Electron Yield.
- Si: K=1849eV; L1=149.7eV; L2=99.8eV; L3=99.4eV; Fe: K=7112eV; L1=844.6eV; L2=719.9eV; L3=706.8eV; Zn: K=9659eV; L1=1196.2eV; L2=1044.9eV; L3=1021.8eV; C: K= 284.2eV; N: K=409.9eV; P: K=2145.5eV; S: K=2472eV.
- The 1s → 3d transition is quantum mechanically forbidden; however, in the view of molecular orbital theory, the metal 3d mix with ligand 2p or 3p orbitals and gain some "p orbital character", weakly alloing the transition.