Titration Fundamentals
- Page ID
- 22129
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)For acid base titrations, a pH indicator or pH meter is used in order to determine whether neutralization has been reached and titration is complete. The information obtained from the process of titration can then be inserted into the equation, \(M_iV_i=M_fV_f\), to determine the concentration of the unknown solution. \(M_i\) and \(M_f\) are the initial and final molarities, and \(V_i\) and \(V_f\) are the initial and final volumes.
Elements of Titration
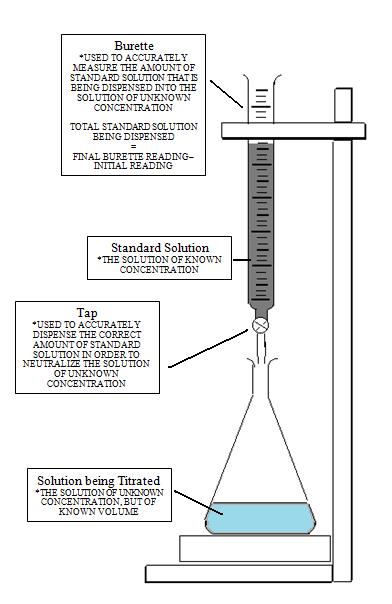
- The standard solution is the solution of known concentration. An accurately measured amount of standard solution is added during titration to the solution of unknown concentration until the equivalence or endpoint is reached. The equivalence point is when the reactants are done reacting.
- The solution of unknown concentration is otherwise known as the analyte. During titration the titrant is added to the analyte in order to achieve the equivalence point and determine the concentration of the analyte.
- The equivalence point is the ideal point for the completion of titration. In order to obtain accurate results the equivalence point must be attained precisely and accurately. The solution of known concentration, or titrant, must be added to the solution of unknown concentration, or analyte, very slowly in order to obtain a good result. At the equivalence point the correct amount of standard solution must be added to fully react with the unknown concentration.
- The end point of a titration indicates once the equivalence point has been reached. It is indicated by some form of indicator which varies depending on what type of titration being done. For example, if a color indicator is used, the solution will change color when the titration is at its end point.
For example, when a color indicator is being used:

To clear confusion, the endpoint and equivalence point are not necessarily equal, but they do represent the same idea. An endpoint is indicated by some form of indicator at the end of a titration. An equivalence point is when the moles of a standard solution (titrant) equal the moles of a solution of unknown concentration (analyte).
Indicators
The use of an indicator is key in performing a successful titration reaction. The purpose of the indicator is to show when enough standard solution has been added to fully react with the unknown concentration. However, an indicator should only be added when necessary and is dependent upon the solution that is being titrated. Therefore, indicators must only be added to the solution of unknown concentration when no visible reaction will occur. Depending on the solution being titrated, the choice of indicator can become key for the success of the titration.
How to Setup a Titration
Materials
- Erlenmeyer flask or beaker
- Excess amount of standard solution (titrant)
- A precisely measured amount of analyte; this will be used to make the solution of unknown concentration
- Indicator
- Calibrated Burette
- Burette Stand
Procedure
- Before beginning the experiment, obtain all necessary materials and clean all necessary items with distilled water.
- Measure out a precise amount of analyte; this will make up the solution of unknown concentration.
- Quantitatively transfer the analyte into a beaker or Erlenmeyer flask. Make sure to rinse all of solid analyte into the beaker or Erlenmeyer flask with distilled water.
- Add additional distilled water until the anlayte is fully dissolved. Measure and record volume of aqueous solution, the process of titration will solve for concentration of this solution.
- Add four to five drops of the appropriate color indicator into the beaker.
- Swirl the beaker in order to mix the aqueous solution of the analyte and the drops of indicator.
- Fill the burette with an excess amount of titrant. The titrant is the standard solution of known concentration and should be in aqueous form.
- Clamp the burette carefully to a burette stand. The tip of the burette should not be touching any surfaces.
- Place the beaker or Erlenmeyer flask containing the aqueous solution of unknown concentration under the burette.
- Record the initial volume of the burette. Make sure to measure at the bottom of the meniscus.
- Turn on the stopcock (tap) of the burette, so that standard solution is added to the beaker. This should cause a color change so be sure to swirl the beaker or Erlenmeyer flask until the color disappears.
- Repeat the above step until the color does not disappear. This means you have reached the endpoint
- Stop when you've reached endpoint, which is the point when the reactant within the solution of unknown concentration has been completely neutralized. You can tell you've reached the endpoint because the color will change.
- Measure and record your final volume of the burette. Calculate the volume of standard solution used by subtracting the initial volume measurement from the final volume measurement of the burette.
- Now perform the necessary calculations in order to obtain the concentration of the unknown solution.
Types of Titrations
The following types of titrations are categorized based on chemical reactions.
Acid-Base Titration Reactions
Titration of acid/base reactions involve the process of neutralization in order to determine an unknown concentration. Acid-Base titrations can be made up of both strong and weak acids or bases. However, in order to determine the unknown concentration of an acid or base, you must add the opposite so that neutralization can be reached. Therefore, an acid of unknown concentration will be titrated using a basic standard solution and a base of unknown concentration will be titrated using an acidic standard solution. Examples include:
- Titration of a Strong Acid with a Strong Base
- Titration of a Weak Acid with a Strong Base
- Titration of a Weak Base with a Strong Acid
- Titration of a Weak Polyprotic Acid
Acid-Base titrations often require the use of some kind of indicator depending on the strength of acid or base that is being titrated. In some cases a weak base or weak acid is used or a ph meter which reads the pH of the solution being titrated. Once the pH of the titrated solution equals seven, either indicated by a change in color or on a pH meter one can determine that titrations is complete.
Redox (Oxidizing-Reduction) Titrations
Another type of titration is the Redox, or Oxidizing-Reducing Titration, which is used to determine the oxidizing or reducing agent in a solution. When performing redox titrations, either the reducing or oxidizing agent will be used as the titrant against the other agent. The purpose of this titration is to determine the transfer of electrons from one substance to the other, similar to that of a redox reaction to determine the reductant or oxidant. The end point of such titrations can be determined by either a color changing indicator or potentiometer.
Combination Reactions Titrations
Combination reaction titrations inclued two different types of titrations:
- One type of combination reaction titrations involve elements of opposite ions being titrated against each other. Therefore, one ion will act as the titrant while the other opposite ion will act as the analyte. However, combination reactions can involve more than two elements that are not necessarily ionic. These types of reactions will sometimes form a precipitate indicating the endpoint, but if not, some type of indicator may need to be added to the solution being titrated.
- Complex-formation Titrations: In these types of titrations the fomation of precipitate may or may not exist. Therefore, these types of titrations require the powerful complexing agent of ethlylenediaminetetraacetic acid (EDTA) or related compounds. For these type of reactions EDTA is used as a titrant becaue it will combine with many different types of cations in order to form a single type of complex. EDTA is most commonly used to determine the metal ions of a solution. However, EDTA should not be confused as being the indicator for these types of reactions, because the indicators are usually organic dyes. In fact EDTA merely acts as an inhibitor because it bonds strongly with the cations of metal, which results in the displacement of the indicator. This is what causes the color change, signifying the endpoint of titration.
Back Titrations
The purpose of back titrating is to return to the endpoint after it was passed. Back titrating should only be used when made necessary. It is often used when the solution being titrated is either too weak or too slow to give a reaction. It is also used if too much titrant was added, and the solution turned too dark. This means the experiment must be done over. The way to back titrate is to add an excess volume of another reactant of known concentration.
A back titration, or reverse titration, is most useful when the endpoint of a normal titration is difficult to identify.
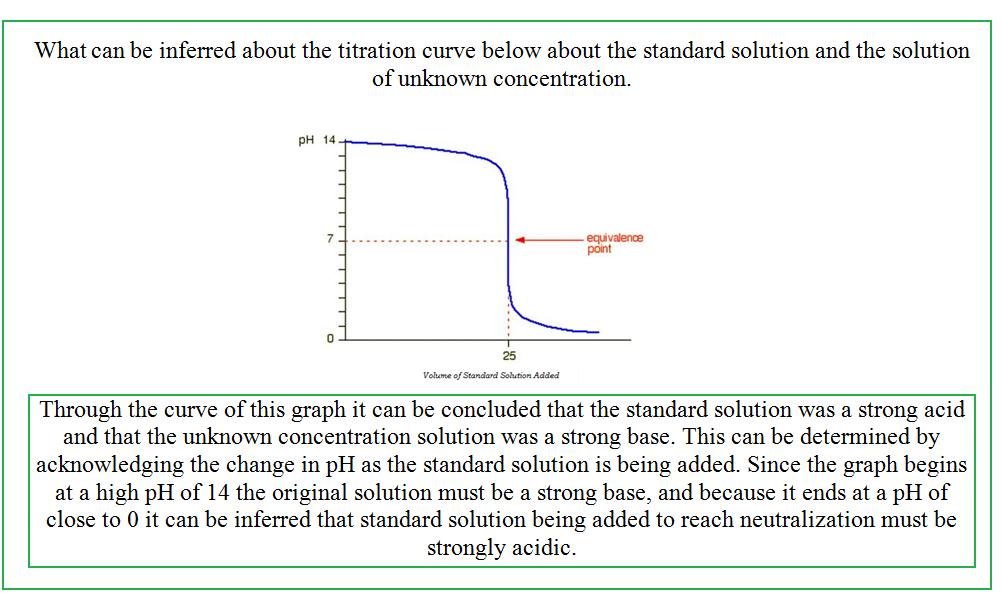
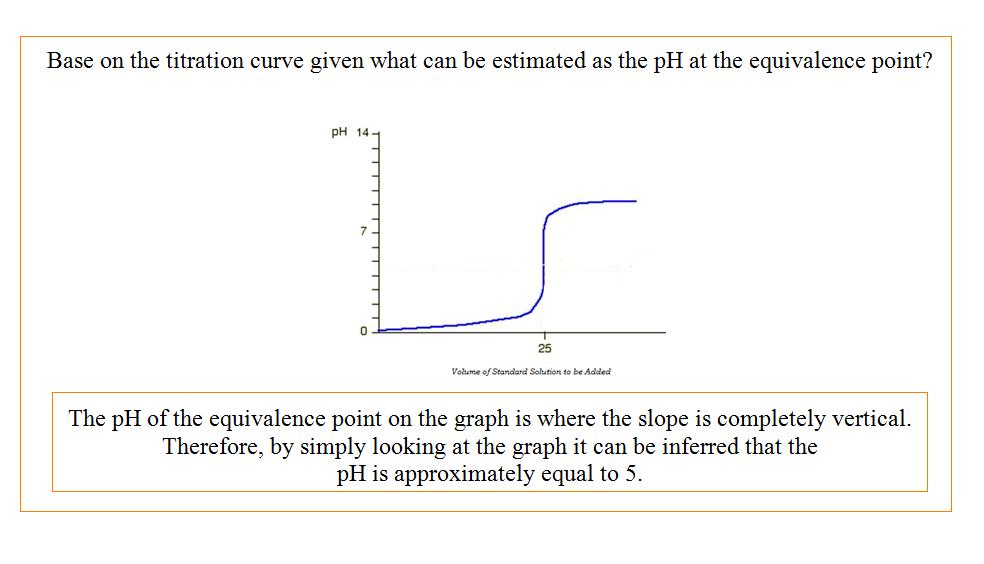
Titration Curves
The graphs of titration curves effectively show the relationship between the pH of the solution of unknown concentration as the standard solution is added to it in order to reach neutralization.
Effects on pH
The pH of the final solution of titration changes as a result of the concentration of the standard solution. Ideally, if the titration has been done precisely and accurately, the final solution of the titration process should be neutralized and have a pH of 7.0. However, this is not always the case. The pH of the final solution often fluctuates depending upon the concentration of the unknown solution and the standard solution that is being added. Therefore, the effects that titration has on pH can best be defined by a generalized trend exhibited by the equivalence points on a titration curve. For more information of pH and pOH click here.
Solving Titration Reactions
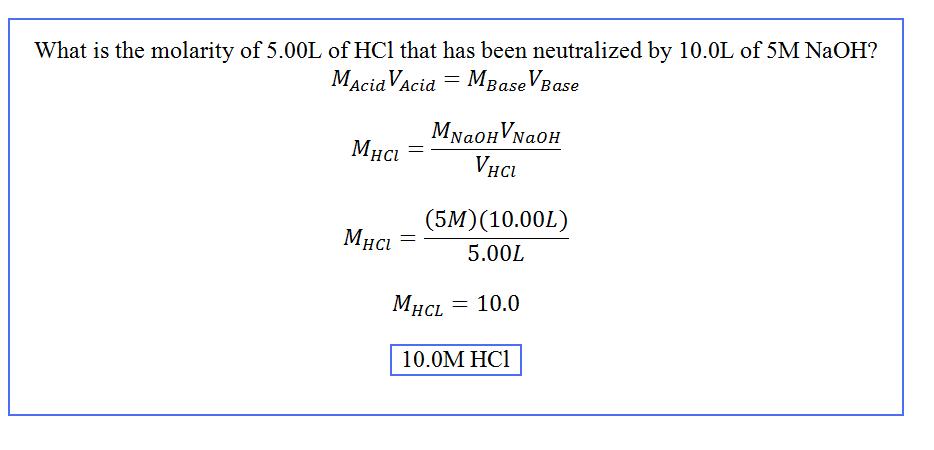
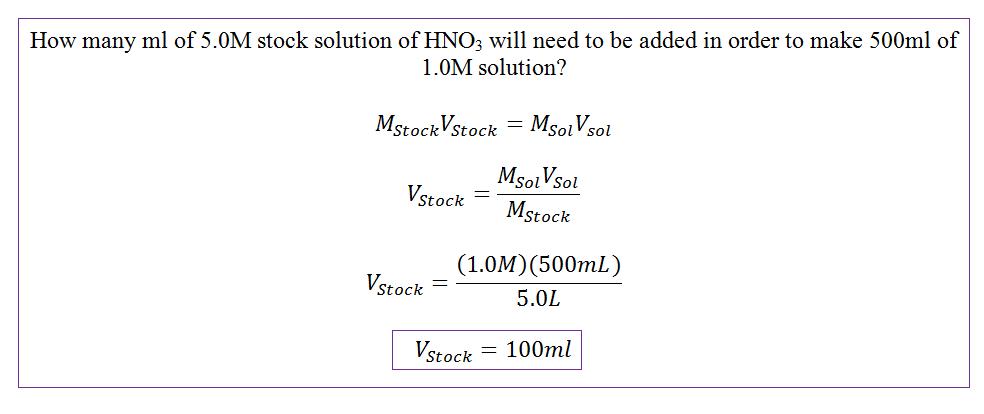
\[ M_1V_1 = M_2V_2 \]
Ideally when performing titration reactions the molarity multiplied by the volume of solution one should equal the molarity multiplied by the volume of solution two. Assume solution one is the standard solution, titrant, and solution two is the solution of unknown concentration, analyte. The volume of the titrant solution can be determined by subtracting the final burette readings from the initial.
An example of the equation for Acid-base titrations:
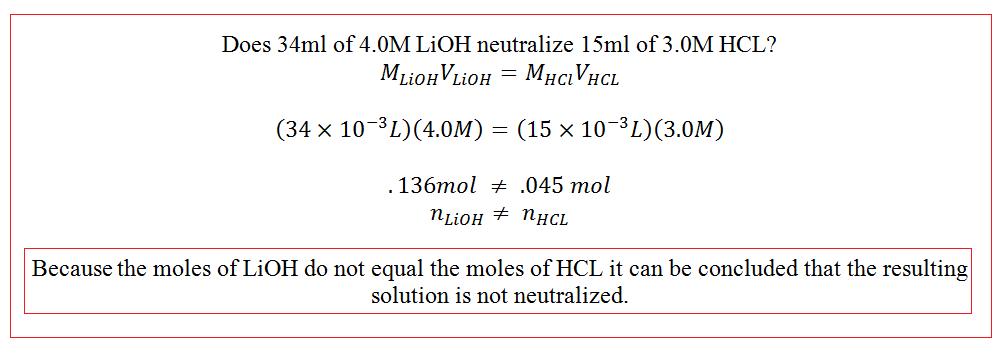
If done correctly, the final solution after titration should be neutralized and contain equal moles of hydroxide and hydrogen ions. So the moles of acid should equal the moles of base:

Outside Links
- Steps to Perform a Titration: http://www.dartmouth.edu/~chemlab/techniques/titration.html
- Pratice Problem: http://chemed.chem.purdue.edu/genchem/probsolv/stoichiometry/acid-base1/
- Titration Curves: http://www.chemguide.co.uk/physical/acidbaseeqia/phcurves.html
- Videos of Titration:http://www.youtube.com/watch?v=YDzzMcrdyB4
- http://group.chem.iastate.edu/Greenb...acid_base.html
References
- Lehrbuch der Chemisch-Analytischen Titrirmethode, Friedrich Mohr, Vieweg, 1862
- The Basics of Chemistry, Meyers, R., Greenwood Press, 2003
- Organic Chemistry, Clayden, J., Warren, S., et al.. Oxford University Press, 2000
- Brady, James E., and John R. Holum. Chemistry The Study of Matter and Its Changes . New York: John WIley & Sons, Inc., 1993. 484- 87. Print.
- Macleod, Anne L., and Edith H. Nason. Chemistry and Cookery . Second ed. New York And London: McGraw Hill Book Company, Inc., 1937. 126-31. Print.
- Miall, L. M, ed. A New Dictionary of Chemistry . Third ed. London, WI: Longmans, Green and Co Ltd , 1961. 549-50. Print.
- Parker, Sybil P, ed. McGraw-Hill Encyclopedia of Chemistry . N.p.: McGraw Hill Book Company, 1983. 1065-70. Print
- Petrucci, Ralph H., William S. Harwood , F. G. Herring, and Jeffrey D. Madura. General Chemistry Principles and Modern Applications. Ninth ed. Upper Saddle River, NJ: Pearson Education, Inc., 2007. 163-67. Print.
- Wilbraham, Anthony C., Dennis S. Staley, Michael S. Matta, and Edward L. Waterman. Prentice Hall Chemistry . Boston: Pearson Education, Inc., 2007. 613-16. Print.
Contributors
- Lauren Xavier (UCD)