6.7: Catalysis
- Page ID
- 499150
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams
Many chemical processes benefit from faster reaction rates. One way to speed up reactions is by increasing the temperature, but high temperatures can cause problems: heat-sensitive compounds may break down, unwanted byproducts can form, and heating can be expensive. Another way to increase the reaction rate is to add a catalyst, a substance that increases the rate of reaction without being consumed itself.
How does a catalyst work?
A catalyst increases the rate of reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower activation energy than the uncatalyzed reaction.
This lower activation energy results in an increase in rate as described by the Arrhenius equation. Because the catalyst lowers the activation energy for both the forward and reverse reactions, it speeds up both directions. Adding a catalyst does not change the equilibrium constant or shift the equilibrium position; the system reaches equilibrium faster (see the later chapter on chemical equilibrium). A catalyzed mechanism generally involves at least two steps: one where the catalyst interacts with a reactant to form an intermediate substance, and one where the intermediate decomposes or reacts with another reactant in one or more steps to regenerate the original catalyst and form the product. In this way, the catalyst is involved in the reaction mechanism but is not consumed by the reaction.
Consider a simple one-step bimolecular reaction: \(X + Y \rightarrow Z \) and its energy diagram in Figure \(\PageIndex{1}\)
In the catalyzed reaction, the same overall reaction proceeds via two steps:
- Step 1: \(X + \text{catalyst} \rightarrow \text{Intermediate}\)
- Step 2: \(\text{Intermediate} + Y \rightarrow Z + \text{catalyst}\)
Key points:
- The lower activation energy of the catalyzed pathway increases reaction rates.
- The catalyst is used and then regenerated, so it does not appear in the overall net reaction.
- A catalyst is different from an intermediate:
A catalyst is consumed as a reactant in one step and produced as a product in another.
An intermediate is first produced as a product in one step and then consumed as a reactant in another.

An important example of catalysis is the destruction of ozone (O₃) in Earth’s stratosphere (10–40 km above the surface). Stratospheric ozone is crucial because it absorbs ultraviolet (UV) radiation from the Sun, preventing harmful UV rays from reaching Earth’s surface, where they can damage living organisms, including humans.
Ozone breaks down when it absorbs UV radiation, following this reaction mechanism:
\[\begin{array}{rcl} &\text{Step 1: (fast)}& \text{O}_3(g) &\xrightarrow{h\nu}& \text{O}_2(g) + \text{O}(g)\\[0.5em] &\text{Step 2: (slow)}& \text{O}_3(g) + \text{O}(g) &\longrightarrow& 2\;\text{O}_2(g)\\[0.5em] &\text{Overall:}& 2\;\text{O}_3(g) &\xrightarrow{h\nu}& 3\;\text{O}_2(g)\end{array} \nonumber \]
Certain substances, such as chlorofluorocarbons (CFCs)—once used in air conditioners and aerosol sprays—release chlorine (Cl) atoms in the stratosphere when exposed to UV light. These Cl atoms act as catalysts, accelerating ozone destruction. For example, the CFC CF₂Cl₂ (dichlorodifluoromethane) absorbs UV light, breaking a C–Cl bond and releasing a Cl atom. This chlorine atom then catalyzes ozone breakdown through the following simplified mechanism:
\[\begin{array}{rcl} &\text{Step 1: (slow)}& \text{Cl}(g) + \text{O}_3(g) &\longrightarrow& \text{O}_2(g) + \text{ClO}(g)\\[0.5em] &\text{Step 2: (fast)}& \text{O}_3(g) + \text{ClO}(g) &\longrightarrow& 2\;\text{O}_2(g)+\text{Cl}(g)\\[0.5em] &\text{Overall:}& 2\;\text{O}_3(g) &\longrightarrow& 3\;\text{O}_2(g)\end{array} \nonumber \]
- In Step 1, a chlorine atom (Cl) reacts with ozone, forming oxygen (O₂) and chlorine monoxide (ClO).
- In Step 2, ClO reacts with another ozone molecule, forming two O₂ molecules and regenerating the Cl atom.
- Since Cl is consumed and then re-formed it is a catalyst. Conversely, ClO is produced and consumed and so it is a reaction intermediate.
A single chlorine atom can destroy up to 100,000 ozone molecules before it reacts with another substance and is removed from the stratosphere. This leads to ozone depletion, reducing Earth's protection from UV radiation and increasing risks like skin cancer, cataracts, and environmental damage.
In 1995, the Nobel Prize in Chemistry was awarded to Paul J. Crutzen, Mario J. Molina, and F. Sherwood Rowland for their groundbreaking work in atmospheric chemistry, specifically their research on the formation and decomposition of ozone.

Mario Molina (born 1943), a Mexican chemist, conducted much of his research at the Massachusetts Institute of Technology (MIT). In 1974, he and F. Sherwood Rowland published a landmark paper in the journal Nature, one of the leading peer-reviewed scientific journals. Their research revealed that chlorofluorocarbons (CFCs)—gases commonly used in refrigeration and aerosol sprays—posed a serious threat to the ozone layer in Earth's upper atmosphere.
The ozone layer plays a critical role in protecting life on Earth by absorbing harmful ultraviolet (UV) radiation from the Sun. Molina and Rowland's research demonstrated that chemical reactions involving CFCs could deplete ozone, leading to the formation of the Antarctic ozone hole. As the ozone layer thins, more UV radiation reaches Earth's surface, increasing the risk of skin cancer, cataracts, and environmental damage.
Their work led to the adoption of the Montreal Protocol in 1987, an international treaty that phased out the production of ozone-depleting chemicals. Today, the treaty has 197 signatory countries, making it one of the most successful environmental agreements in history. The ozone layer is now recovering, demonstrating the global impact of their research.
Homogeneous Catalysts
A homogeneous catalyst is in the same phase (solid, liquid, or gas) as the reactants. It interacts with a reactant to form an intermediate, which then breaks down or reacts with another substance to regenerate the original catalyst and form the final product.
For example, chlorine atoms (Cl) act as homogeneous catalysts in ozone decomposition because all reactants are in the gas phase.
Heterogeneous Catalysts
A heterogeneous catalyst is in a different phase than the reactants. These catalysts are often solid materials with large surface areas, where the reaction takes place. They speed up reactions by providing a surface where reactant molecules can interact and react more efficiently than they would in the gas or solution phase alone.
One well-known heterogeneous catalyst is nickel (Ni), which is used to hydrogenate carbon–carbon double bonds in polyunsaturated fats and oils. This reaction converts unsaturated fats (containing double bonds) into saturated fats (containing only single bonds). An example of this process is the hydrogenation of ethylene, as shown in Figure \(\PageIndex{3}\).

Figure \(\PageIndex{3}\): There are four steps in the Ni catalysis of the reaction \(\ce{C2H4 + H2 ⟶ C2H6}\):
a) Adsorption of Hydrogen: Hydrogen gas (H2) binds to the nickel surface, breaking the H–H bonds and forming Ni–H bonds.
b) Adsorption of Ethylene: Ethylene (C2H4) also binds to the nickel surface, weakening the C=C double bond and forming Ni–C bonds.
c) Surface Diffusion & Reaction: Hydrogen atoms migrate across the surface and react with ethylene, forming new C–H bonds.
d) Desorption: The final product, ethane (C2H6), is released because it is not strongly attracted to the nickel surface.
Other significant industrial processes that involve the use of heterogeneous catalysts include the preparation of sulfuric acid, the preparation of ammonia, the oxidation of ammonia to nitric acid, and the synthesis of methanol, CH3OH. Heterogeneous catalysts are also used in the catalytic converters found on most gasoline-powered automobiles (Figure \(\PageIndex{4}\)).
Catalytic converters reduce toxic emissions from gasoline engines by using platinum-rhodium catalysts to speed up reactions that make exhaust gases less harmful.
Modern three-way catalytic converters help convert pollutants into safer substances:
\[\ce{2NO2}(g)⟶\ce{N2}(g)+\ce{2O2}(g)\\
\ce{2CO}(g)+\ce{O2}(g)⟶\ce{2CO2}(g)\\
\ce{2C8H18}(g)+\ce{25O2}(g)⟶\ce{16CO2}(g)+\ce{18H2O}(g) \nonumber \]

Figure \(\PageIndex{4}\) shows how a catalytic converter functions. As exhaust gases enter, an air pump adds extra oxygen to promote complete combustion. The metal catalyst provides a surface where the reactions take place, converting toxic emissions into cleaner gases. The final exhaust contains mostly nitrogen, carbon dioxide, and water vapor, significantly reducing pollution. To work efficiently, catalytic converters must reach high temperatures. Many modern systems use electric preheaters to activate the catalyst faster, ensuring effective emissions reduction even before the engine warms up.
By converting harmful gases into safer ones, catalytic converters help reduce air pollution and protect human health.
Enzymes: Biological Catalysts
Millions of chemical reactions occur within our cells every minute, but without catalysts, most would be too slow to sustain life. Biological catalysts, known as enzymes, dramatically increase reaction rates, often by factors of 10⁶ to 10²⁰.
For example, the breakdown of carbonic acid (H2CO3) in the lungs:
H2CO3(aq) → CO2(g) + H2O(l)
occurs at only ~10⁻⁷ M/s at room temperature—far too slow to meet the body’s needs. The enzyme carbonic anhydrase speeds up this reaction by over a million times, ensuring rapid CO2 production and removal.
Like all catalysts, enzymes lower activation energy by forming a lower-energy transition state. The reactant(s) in an enzyme-catalyzed reaction are called substrates. Enzymes interact with substrates through hydrogen bonds, ionic attractions, or dipole-dipole interactions. Many enzymes recognize only one specific substrate or a closely related group of molecules. Even a small structural change (such as adding a single methyl group, -CH3) can prevent binding.
Enzymes are usually large protein molecules, but only a small region, the active site, binds the substrate and carries out the reaction. The unique shape of the active site allows enzymes to recognize and act on specific molecules, ensuring precise control over biochemical reactions.
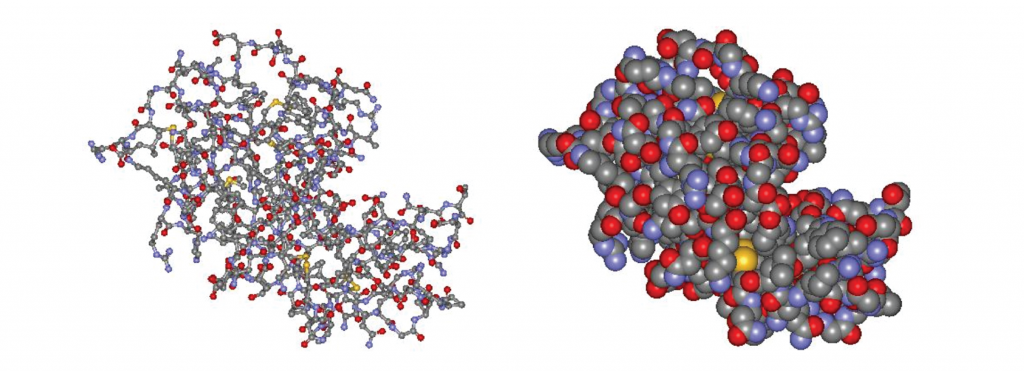 Figure \(\PageIndex{5}\) Structure of the enzyme lysozyme. Left: ball-and-stick model. Right: space filling model. The cleft in the middle right is the active site, where substrate molecules fit. (Structures from http://lysozyme.co.uk.)
Figure \(\PageIndex{5}\) Structure of the enzyme lysozyme. Left: ball-and-stick model. Right: space filling model. The cleft in the middle right is the active site, where substrate molecules fit. (Structures from http://lysozyme.co.uk.)
Models of Enzyme Function
In 1890, Emil Fischer proposed the lock-and-key model, where the substrate fits precisely into the enzyme’s active site, like a key fitting into a lock.
In 1958, Daniel E. Koshland Jr. refined this idea with the induced-fit model. He suggested that the enzyme and substrate adjust their shapes upon binding, improving the fit. This explains both enzyme specificity and how enzymes speed up reactions.
- As the substrate interacts with the enzyme, it distorts (atoms shift, bonds stretch, and reactive groups move closer together) to a structure closer to the transition state. This rearrangement lowers the activation energy, accelerating the reaction.
- Only molecules with the correct shape and functional groups can properly bind to the enzyme's active site.
-

Figure \(\PageIndex{6}\): (a) in the lock-and-key model, the enzyme’s active site has a fixed shape that matches the substrate exactly. (b) In the induced-fit model, the active site is somewhat flexible, adjusting its shape to accommodate the substrate.
Enzyme Kinetics: Michaelis-Menten Mechanism
The Michaelis-Menten mechanism describes how enzymes catalyze reactions through a two-step process. An enzyme (E) first binds with a substrate (S) to form an enzyme-substrate complex (ES), which then breaks down to release the product (P) while regenerating the enzyme. The overall reaction is:
S → P
with the simple two-step mechanism:
\[\begin{array}{rcl}&\text{step 1: (fast)}&\;\;\;\;\;\;\;\;\;\;\;E + S &\xrightleftharpoons[k_{-1}]{k_1}& ES\\[0.5em] &\text{step 2: (slow)}&\;\;\;\;\;\;\;\;\;\;\;ES &\xrightarrow{k_2}& P + E\end{array} \nonumber \]
The second step is rate determining.
Enzyme kinetics studies are typically carried out by measuring the reaction rate as a function of the concentration of substrate and the concentration of enzyme. The rate of the catalyzed reaction can be measured by observing the rate of the production of the product:
\[rate=\dfrac{\Delta[P]}{\Delta t} \nonumber \]
Initially, as the substrate concentration increases, the reaction rate also increases because more enzyme-substrate complexes (ES) are formed. In this region, the reaction follows first-order kinetics, meaning the rate is proportional to [S]:
rate = k[S].
However, once all enzyme active sites are occupied, adding more substrate does not increase the rate. At this point, the reaction reaches a maximum rate and becomes zero-order in [S], meaning the rate is independent of substrate concentration (Figure \(\PageIndex{7}\)).

Enzymes play a crucial role in metabolism, and a deficiency in certain enzymes can lead to serious health conditions. Glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common enzyme deficiency in humans. This enzyme, shown in Figure \(\PageIndex{8}\), is essential for producing NADPH, a molecule that protects red blood cells from oxidative damage.
When G6PD is deficient, red blood cells lose their ability to maintain glutathione, an antioxidant that prevents damage to proteins like hemoglobin. Without enough glutathione, hemoglobin breaks down, leading to jaundice and other complications.
People with G6PD deficiency must avoid certain foods and medications that can trigger red blood cell damage.
Summary
Catalysts speed up reactions by providing a lower-energy pathway, but they are not consumed in the reaction.
In heterogeneous catalysis, catalysts provide a solid surface where reactants bind (adsorption).
In homogeneous catalysis, catalysts and reactants exist in the same phase.
Enzymes are biological catalysts that dramatically increase reaction rates and are highly specific to their substrates.
In enzyme-catalyzed reactions, the substrate binds to the enzyme’s active site, forming a temporary enzyme-substrate complex before converting into the product.
Footnotes
- “The Nobel Prize in Chemistry 1995,” Nobel Prize.org, accessed February 18, 2015, Nobel Prizes Chemistry [www.nobelprize.org].


