1.1.1: Coulomb's Law
- Page ID
- 195509
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Coulomb's Law
Electrons are negatively (-) charged and are attracted to the positive (+) charge of a nucleus. Electrons in a multi-electron atom also repel each other. Coulomb's Law (from classical physics) can be used to describe the attraction and repulsion between any charged particles, including atomic particles.
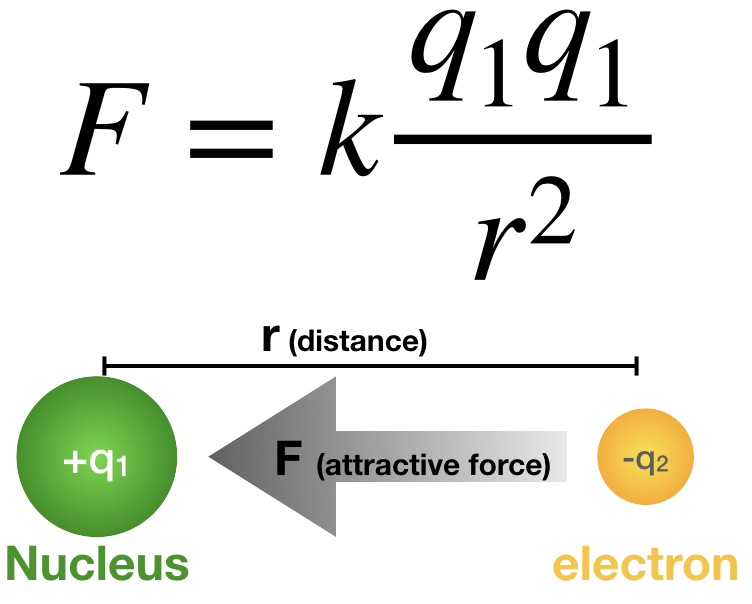
Coulomb's law is defined as:
\[ F=k \dfrac{q_1q_1}{r^2} \label{1}\]
|
Figure \(\PageIndex{1}\). Coulomb's law applied to the attractive force between a nucleus and an electron. (CC-BY-NC-SA; Kathryn Haas) |
Where \(F\) is the force, \(k\) is Coulomb's constant, \(q_1\) and \(q_2\) are the charges on the two particles, and \(r\) is the distance between the particles. This relationship shows that the attractive or repulsive force between two particles is dependent on the distance (r) between the particles, and the sign and magnitude of charges on either particle (q). When the charges have opposite sign, the force is attractive (negative F), while if both charges have the same sign, the force is repulsive (positive F). The magnitude of the force is directly related to the magnitude of charge on the particles (higher charge yields stronger force) and indirectly related to the distance between the particles (large distance yields weaker force).
Coulomb's law describes atoms and orbital energies: In most cases, the attractive force between an electron and a nucleus is much stronger than the repulsive force between electrons. We will focus on the more important attractive force between electrons and nuclei.
In an atom, the most important factors that influence the force, as calculated by Coulomb's equation, are the nuclear charge and the distance from the nucleus to the electron of interest. The closer an electron is to a nucleus, the stronger the attractive force (i.e. the more negative F becomes). Likewise, the greater the nuclear charge, the stronger the attractive force. Regarding distance (\r\), the shell number is the most important factor and the subshell is another factor (this will be further explained in this unit on the section Shielding and Penetration).
Coulomb's law can also be applied to describe the repulsive force between two electrons. However, it does not fully account for the additional, and more significant interplay between multiple electrons and nuclei that occur in multi-electron atoms. Coulomb's Law is limited to the description of a two-particle system. Therefore, this principle works well for describing a hydrogen atom, but Coulomb's law is only part of the story for multi-electron atoms.
Why does a 1s electron have a higher ionization energy than a 3s electron?
- Answer
-
As shell number increases, the distance of the electron from the nucleus increases. In n = 1 shell, electrons are at the closest possible distribution to the nucleus. In n = 3, electrons in the s orbital are much farther from the nucleus on average than the entire 1s orbital. (We can see the probable distance of electrons using the radial distribution functions). Coulomb's law shows that opposite charges have a stronger attractive force when they are close. Thus, the 1s electron is more attracted to the nucleus than a 3s electron, and the 1s electron is more difficult to remove because it is more strongly attracted to the nucleus.
Apply Coulomb's Law to explain why the ground state of H is 1s1 rather than 2s1.
- Answer
-
The force (F) in Coulomb's Law is analogous to energy (E) of an electron when that electron is interacting with a nucleus. As two particles are brought closer together, the value of F (force or energy) becomes more negative, which means that the particles are more attracted to each other.
In the ground state, electrons are in the lowest energy configuration, which is their most stable configuration. Electrons (-) are stabilized (energy is lowered / more negative) by interactions with a nucleus (+). The closer an electron is to the nucleus, the more attracted it is, more stabilized it is, and more its energy is decreased.