17.1: Naming Alcohols and Phenols
- Page ID
- 36345
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- identify an alcohol as being primary, secondary or tertiary, given its structure, its IUPAC name or its trivial name.
- write the IUPAC name of an alcohol or phenol given its Kekulé, condensed or shorthand structure.
- draw the structure of an alcohol or phenol given its IUPAC name.
- identify a number of commonly occurring alcohols (e.g., benzyl alcohol, tert‑butyl alcohol) by their trivial names.
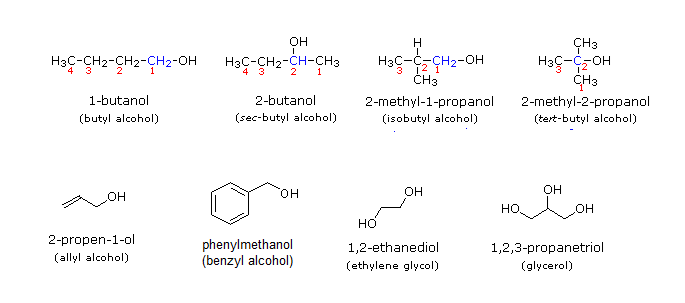
The following are common names of some alcohols (with IUPAC name).
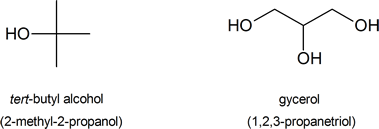
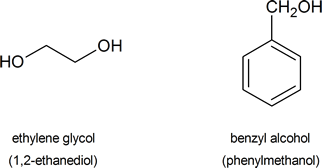
Alcohol Classifications
Alcohols can be classified as primary (1o), secondary (2o), or tertiary (3o) depending on the number of alkyl substituents attached to the carbon bonded to the O-H group.
Primary alcohols
In a primary (1°) alcohol, the carbon which carries the -OH group is only attached to one alkyl group. Some examples of primary alcohols include:
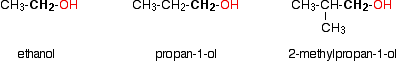
Notice that it doesn't matter how complicated the attached alkyl group is. In each case there is only one linkage to an alkyl group from the CH2 group holding the -OH group. There is an exception to this. Methanol, CH3OH, is counted as a primary alcohol even though there are no alkyl groups attached to the carbon with the -OH group on it.
Secondary alcohols
In a secondary (2°) alcohol, the carbon with the -OH group attached is joined directly to two alkyl groups, which may be the same or different. Examples:
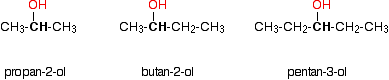
Tertiary alcohols
In a tertiary (3°) alcohol, the carbon atom holding the -OH group is attached directly to three alkyl groups, which may be any combination of same or different. Examples:
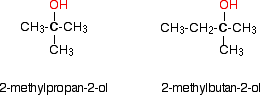
Indicate if the following molecules are 1o, 2o, or 3o alcohols:
1)
a)
b) (CH3)3COH
c) CH3CH2CH2OH
d)
- Answer
-
1)
a) Secondary
b) Tertiary
c) Primary
d) Secondary
Naming Alcohols
The IUPAC naming of alcohols is based off the name of the parent alkane chain:
- The longest chain containing the hydroxyl group (OH) is considered the parent chain. Remove the final -e from the parent alkane chain name and add the suffix -ol.
- Number the parent alkane chain such that the hydroxyl group get the lowest possible number. Older IUPAC rules origianlly place the hydroxyl group number before the name of the parent chain. However, the newer rules places the number before the -ol suffix.
- Number the substituents according to their position on the parent chain. Then list the substituents in alphabetical order.
4. When naming a cyclic structure with a hydroxyl group, the -OH is assumed to be on the first carbon.
5. When multiple alcohols are present use di, tri, et.c before the ol, after the parent name. Also, when a prefix is used the -e is not removed from the parent chain name ex. 2,3-hexanediol
6. When an alkene and alcohol are present in a molecule it is named as follows (location of the alkene)-(prefix for the parent chain + en)-(location of the hydroxyl)-ol
Common Names of Alcohols
The common system of naming is often used when the alcohol only contains a few carbons. As discussed in Section 3-3, the common system names alcohols as if the hydroxyl group (-OH) is attached to a single substituent with the word alcohol added at the end (Name of the substituent + Alcohol). Also, some simple alcohols are given their own generic name such ethylene glycol or glycerol.
Naming phenols
Phenols are named using the rules for aromatic compounds discussed in Section 15-1. Note that -phenol is used as the ending rather than -benzene.
Exercises
Give IUPAC names for the following structures.
- Answer
-
Name the following structures.
- Answer
-
Draw and name all the alcohol isomers of C3H9O
- Answer
-
Add texts here. Do not delete this text first.
Oleic acid, a commonly occurring fatty acid in vegetable oils, has the following structure. Name the compound, making sure to give the correct alkene geometry.
- Answer
-
(9Z)-Octadec-9-enoic acid
Creosols are naturally occurring compounds used building blocks for many molecules, they occur as three different isomers. Name each of the following isomers.
- Answer
-

