17.0: Introduction to Alcohols and Phenols
- Page ID
- 36344
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- describe the structural differences among alcohols, phenols and enols.
- write equations describing the industrial preparation of the two simplest alcohols: methanol and ethanol.
Make certain that you can define, and use in context, the key terms below.
- alcohol
- enol
- phenol
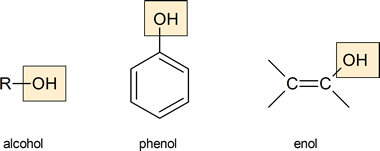
Alcohols and phenols are organic compounds with at least one hydroxyl group attached to a saturated or an aryl carbon, respectively. Enols are a related third class of compounds, with the hydroxyl group attached to a vinylic carbon. We will discuss enols in more detail in Chapter 22.
The reading correctly points out the toxicity of methanol, and describes the adverse effects of its consumption. These effects have not been exaggerated; you may recall reading about the deaths of six residents of Peerless Lake, Alberta, in 1986, brought about by drinking photocopier fluid which contained methanol (or methyl hydrate as it is often called in press reports). In 2000, more than 100 people died in El Salvador after black marketeers sold discarded liquor bottles that had been refilled with a methanol mixture. Indeed the problem has repeated itself globally and so often that in 2014 the World Health Organization released an information note warning of methanol poisoning outbreaks which “occur when methanol is added to illicitly‑ or informally‑produced alcoholic drinks.”
Almost everyone is aware that the alcohol present in alcoholic beverages is ethanol (also called ethyl alcohol or grain alcohol). However, many people do not realize that in its pure state, or in solutions of high concentration, this substance is poisonous. In the laboratory one may find containers labelled “absolute ethanol,” “95% ethanol” and “denatured ethanol.” The acquisition of ethanol by laboratories, and its subsequent disposal, is carefully monitored by provincial authorities. On no account should one consider drinking laboratory ethanol, even after it has been diluted to a concentration equivalent to that found in beer. Denatured alcohol is ethanol to which appropriate quantities of poisonous or nauseating substances (such as methanol) have been added.
A third commonly encountered alcohol, isopropyl alcohol (“rubbing alcohol” or 2‑propanol), is also toxic. It has the ability to kill germs and has a temporary lubricating effect during the rubbing process. Unlike methanol, 2‑propanol is not absorbed through the skin; therefore it poses less of a health hazard.
The use of alcohols as fuels is well established. In 2010, the Canadian federal government joined several of the provinces in requiring that all gasoline must include an average of five per cent ethanol. Some gasoline producers, notably Husky/Mohawk, average ten per cent ethanol content in their products. Some especially modified vehicles can use fuel that consists of 85 per cent ethanol (E85).
A phenol is an organic compound in which a hydroxyl group is directly bonded to one of the carbon atoms of an aromatic ring.
Until the late nineteenth century, a person undergoing surgery had to face the fact that he or she might suffer the consequences of what we now know to be bacterial infection, contracted during the course of the operation. The physicians of the time did not know that bacteria existed, and had no way to counter the problems that bacteria caused. In 1867, Joseph Lister, who had learned of the existence of bacteria as a result of research done by Louis Pasteur, began using solutions of phenol to clean wounds and surgical instruments. The phenol solution was an effective antiseptic, killing bacteria, and as a result, a patient’s chances of surviving surgery improved greatly. Phenol itself was rather strong for these purposes—it burns healthy tissue—and substitutes were eventually found. One such substitute, used today in throat lozenges and mouthwashes, is 4‑n‑hexylresorcinol.
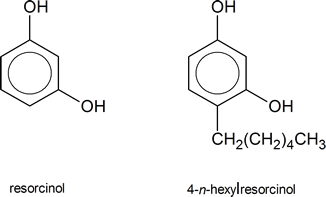
Alcohols
Molecules of alcohols contain one or more hydroxyl groups (OH groups) substituted for hydrogen atoms along the carbon chain. The structure of the simplest alcohol, methanol (methyl alcohol), can be derived from that of methane by putting an OH in place of one of the H’s:
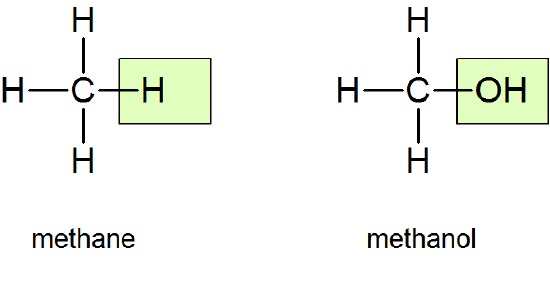
The name, too, is derived from the name methane by replacing the final e with ol (for alcohol). The general formula for an alcohol may be written as R—OH, where R represents the hydrocarbon (alkane) portion of the molecule and is called an alkyl group. In methanol, R is the methyl group CH3.
Methanol is also called wood alcohol because it can be obtained by heating wood in the absence of air, a process called destructive distillation. Methanol vapor given off when the wood is heated can be condensed to a liquid by cooling below its boiling point of 65°C. The effect of polarity and especially hydrogen bonding due to the OH group is evident when this is compared with the temperature of –85°C at which ethane, C2H6, boils. Both molecules contain 18 electrons and are nearly the same size, and so London forces should be about the same, but the OH group in one methanol molecule can form strong hydrogen bonds with an OH in another molecule. Methanol is an important industrial chemical—nearly 3 × 1010 kg was produced worldwide in 2003[1]. Some was made by destructive distillation, but most was synthesized from hydrogen and carbon monoxide:
\[\ce{2H_{2} (g) + CO (g) \longrightarrow CH_{3}OH(l)} \nonumber \]
This reaction is carried out at pressures several hundred times normal atmospheric pressure, using metal oxides as catalysts. Methanol is mainly used to make other compounds from which plastics are manufactured, but some is consumed as fuel in jet engines and racing cars. Methanol is also a component of nonpermanent antifreeze and automobile windshield-washer solvent.
The second member of the alcohol family is ethanol (ethyl alcohol)― the substance we commonly call alcohol. Ethanol is also known as grain alcohol because it is obtained when grain or sugar ferments. Fermentation refers to a chemical reaction which is speeded up by enzymes and occurs in the absence of air. (Enzymes, catalysts which occur naturally in yeasts and other living organisms, are discussed in more detail elsewhere.)
Ethanol can also be synthesized by adding H2O to ethene, obtained during petroleum refining:
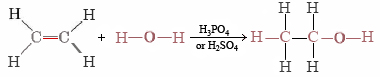
This is a typical example of an addition reaction. The H and OH from H2O are added to the ethene molecule and held there by electrons made available when one-half of the double bond breaks.
Ethanol is used as a solvent, in some special fuels, in antifreeze, and to manufacture a number of other chemicals. You are probably most familiar with it as a component of alcoholic beverages. Ethanol makes up 3 to 6 percent of beer, 12 to 15 percent of most wines, and 49 to 59 percent of distilled liquor. (The “proof” of an alcoholic beverage is just twice the percentage of ethanol.) Alcohol’s intoxicating effects are well known, and it is a mild depressant. Prolonged overuse can lead to liver damage. Methanol also produces intoxication but is much more poisonous than ethanol—it can cause blindness and death. Denatured alcohol is ethanol to which methanol or some other poison has been added, making it unfit for human consumption. Most of the ethanol not used in alcoholic beverages is denatured because in that form its sale is taxed at a much lower rate.
Phenols
Compounds in which a hydroxyl group is bonded to an aromatic ring are called phenols. The chemical behavior of phenols is different in some respects from that of the alcohols, so it is sensible to treat them as a similar but characteristically distinct group. A corresponding difference in reactivity was observed in comparing aryl halides, such as bromobenzene, with alkyl halides, such as butyl bromide and tert-butyl chloride. Thus, nucleophilic substitution and elimination reactions were common for alkyl halides, but rare with aryl halides. This distinction carries over when comparing alcohols and phenols, so for all practical purposes substitution and/or elimination of the phenolic hydroxyl group does not occur.

