20.S: Carboxylic Acids and Nitriles (Summary)
- Page ID
- 207034
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Concepts & Vocabulary
20.0 Chapter Objectives and Introduction to Carboxylic Acids
- Carboxylic acids are defined as molecules that have a hydroxyl group bonded to a carbonyl group (RCOOH).
- Fatty acids are naturally occurring carboxylic acids that are important components of lipids.
- Fatty acids can either be saturated (all carbon-carbon bonds are single bonds) and unsaturated (one or more carbon-carbon double bonds).
- Several other functional groups can be prepared from carboxylic acids and are considered derivatives (acyl halides, anhydrides, esters, amides and nitriles).
20.1 Naming Carboxylic Acids and Nitriles
- Carboxylic acids are named following IUPAC rules with ending -oic acid.
- When carboxylic acids are named as an attachment to a ring, add -carboxylic acid to the name of the cyclic compound.
- Nitriles are named following IUPAC rules with the ending -nitrile or -carbonitrile.
20.2 Structure and Properties of Carboxylic Acids
- Carboxylic acids have trigonal planar shape around the carbonyl carbon and due to resonance structures, all the atoms of the functional group are co-planar.
- Conjugation in carboxylic acids increases acidity of the hydroxyl hydrogen compared to alcohols. The conjugate base (carboxylate) is stabilized by resonance.
- Carboxylic acids form hydrogen bonded dimers yielding higher boiling points than for other molecules of similar size.
- Small carboxylic acids are often soluble in water due to hydrogen bonding. Larger carboxylic acids are often slightly soluble in water, though when converted to a carboxylate salt by treating with base, become water soluble.
20.3 Biological Acids and the Henderson-Hasselbalch Equation
- Carboxylic acids are fully protonated in acidic solutions.
- Carboxylic acids are fully dissociated in basic solutions.
- The Henderson-Hasselbalch equation can be used to relate pH, pKa and ratio of concentrations of acid and conjugate base.
20.4 Substituent Effects on Acidity
- Withdrawing groups (through inductive or resonance effects) can stabilize carboxylates, increasing acidity of carboxylic acids.
- Donating groups (through inductive or resonance effects) destabilize carboxylates, decreasing acidity of carboxylic acids.
- Nearly all substituents in ortho positions increase acid strength whether donating or withdrawing, this is called the ortho-effect.
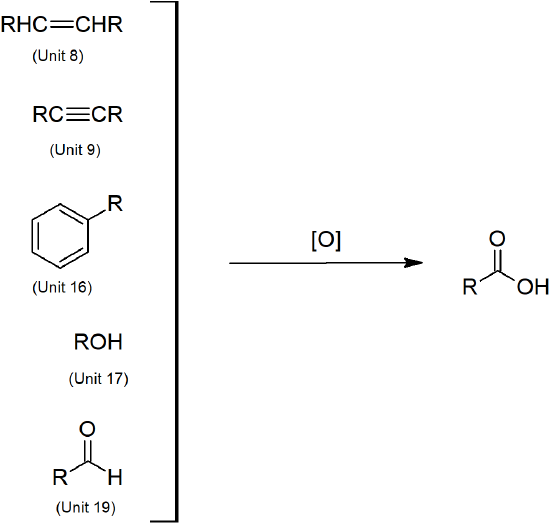
20.5 Preparing Carboxylic Acids
- Carboxylic acids can be prepared through oxidation of several functional groups including: 1o alcohols, aldehydes, alkyl arene side chains, and alkenes.
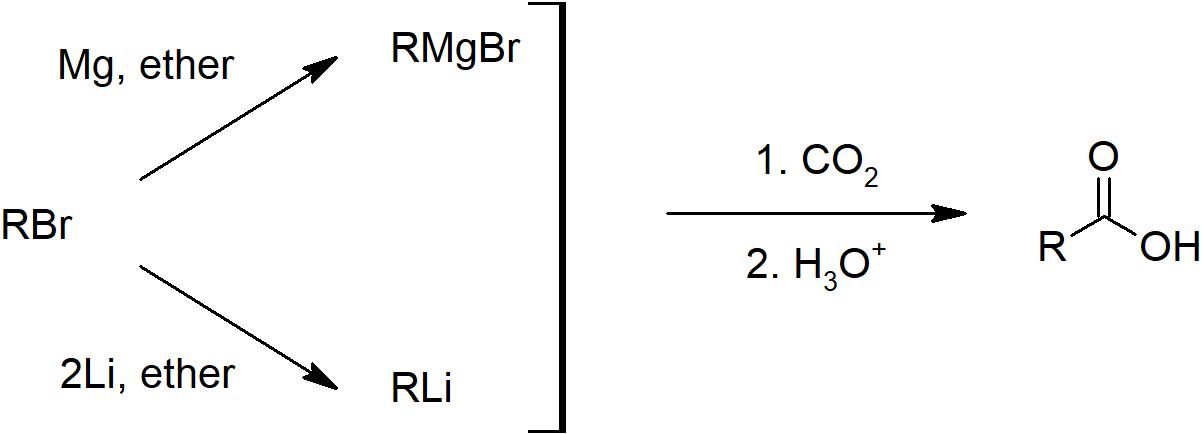
- Carboxylic acids can also be prepared by carboxylation of Grignard reagents and hydrolysis of nitriles.
20.6 Reactions of Carboxylic Acids: An Overview
- There are four main categories of carboxylic acid reactions:
- Acid/base reaction (salt formation)
- Nucleophilic acyl substitution
- Reduction
- Substitution alpha to the carbonyl
- Nitriles have linear geometry due to the sp hybrid orbitals on carbon and nitrogen.
- Nitriles are less basic than amines.
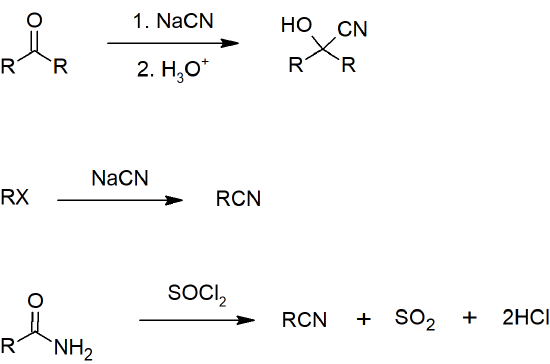
- Nitriles can be prepared from aldehydes, ketones, or amides.
- Nitriles react similarly to carbonyl compounds where nucleophiles will attack the carbon end of the nitrile.
- Nitriles can be hydrolyzed to carboxylic acids under basic or acidic conditions.
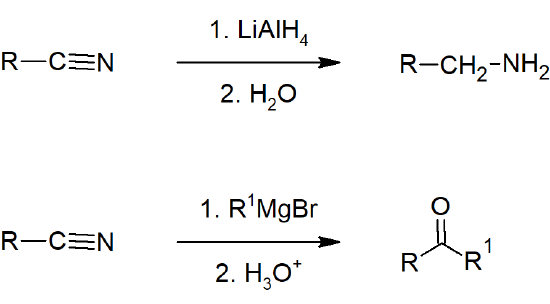
- Nitriles can be reduced to amines by hydride reduction.
- Nitriles can be converted to aldehyde by reaction with DIBALH.
- Nitriles can be converted to ketones by reaction with organometallic compounds.
20.8 Spectroscopy of Carboxylic Acids and Nitriles
- IR of carboxylic acids typically show a very strong and broad OH stretch from about 2500 to 3300 cm-1 as well as a strong carbonyl stretch around 1710 cm-1.
- 1H NMR of carboxylic acids show the OH proton between 10-12 ppm as well as hydrogens on carbon adjacent to the carbonyl around 2-3 ppm.
- 13C NMR of carboxylic acids show the carbonyl carbon at 160-180 ppm.
- Mass spectra of carboxylic acids often have fragments for OH (loss of 17) and COOH (loss of 45).
- The most identifying characteristic of IR of nitriles is the CN triple bond stretch which appears near 2250 cm-1.
- 1H NMR of nitriles show protons on the carbon next to the nitrile at around 2-3 ppm.
- 13C NMR of nitriles show the nitrile carbon in the 115-120 ppm range.
- Mass spectra of nitriles will often not have a visible molecular ion, but instead exhibit a strong M-1 peak.
Skills to Master
- Skill 20.1 Name carboxylic acids and nitriles using IUPAC rules.
- Skill 20.2 Draw the structure of carboxylic acids and nitriles from the IUPAC name.
- Skill 20.3 Describe the geometries and approximate bond angles of carboxylic acids and nitriles.
- Skill 20.4 Explain the acidity of carboxylic acids based on conjugate base stabilization.
- Skill 20.5 Explain physical properties of carboxylic acids.
- Skill 20.6 Describe the structure of carboxylic acids in buffered solutions.
- Skill 20.7 Use the Henderson-Hasselbach equation to relate pH, pKa, and acid/conjugate base concentrations.
- Skill 20.8 Explain inductive effects on acidity.
- Skill 20.9 Explain relative acidity of aromatic acids.
- Skill 20.10 Draw mechanisms for preparing carboxylic acids including:
- Oxidations
- Carboxylation of Grignard reagents
- Hydrolysis of Nitriles
- Skill 20.11 List the four categories of carboxylic acid reactions.
- Skill 20.12 Draw reactions for preparing nitriles including formation from:
- aldehyde or ketone
- 1o amide
- Skill 20.13 List general reactions of nitriles.
- Skill 20.14 Draw mechanisms for reactions of nitriles including:
- Hydrolysis to acids (acidic and basic mechanisms)
- Reduction to 1o amines
- Conversion to aldehyde by reaction with DIBALH.
- Conversion to ketones by reaction with organometallic compounds.
- Skill 20.15 Plan a synthesis of a ketone from a nitrile.
- Skill 20.16 Use IR, NMR and MS to identify carboxylic acids and nitriles.
Summary of Reactions
Carboxylic Acid Preparation

Nitrile Preparation
Reaction of Nitriles