20.1: Naming Carboxylic Acids and Nitriles
- Page ID
- 36550
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- write the IUPAC name of a carboxylic acid, given its Kekulé, condensed or shorthand structure.
- draw the condensed or shorthand structure of a carboxylic acid, given its IUPAC name.
- draw the structure of the following carboxylic acids, given their trivial names: formic acid and acetic acid.
- provide an acceptable name for a nitrile of given structure.
- draw the condensed or shorthand structure of a nitrile, give either its trivial or IUPAC name.
Make certain that you can define, and use in context, the key terms below.
- carboxylic acid
- nitrile
You need not memorize all the trivial names listed in the table, just remember the two names identified in Objective 3, above.
Carboxylic Acids, RCO2H
The IUPAC system of nomenclature assigns a characteristic suffix to these classes. The –e ending is removed from the name of the parent chain and is replaced -oic acid. Since a carboxylic acid group must always lie at the end of a carbon chain, it is always is given the #1 location position in numbering and it is not necessary to include it in the name.
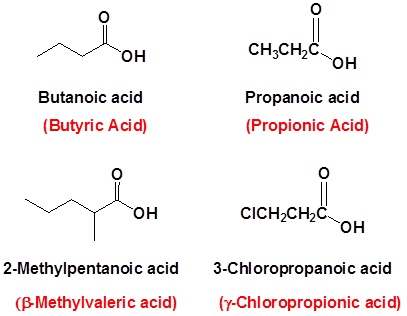
Many carboxylic acids are called by the common names. These names were chosen by chemists to usually describe a source of where the compound is found. In common names of carboxylic acids, carbon atoms near the carboxyl group are often designated by Greek letters. The atom adjacent to the carbonyl function is alpha, the next removed is beta and so on.
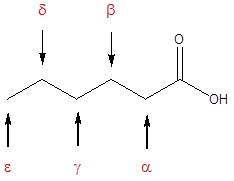
| Formula | Common Name | Source | IUPAC Name | Melting Point | Boiling Point |
|---|---|---|---|---|---|
| HCO2H | formic acid | ants (L. formica) | methanoic acid | 8.4 ºC | 101 ºC |
| CH3CO2H | acetic acid | vinegar (L. acetum) | ethanoic acid | 16.6 ºC | 118 ºC |
| CH3CH2CO2H | propionic acid | milk (Gk. protus prion) | propanoic acid | -20.8 ºC | 141 ºC |
| CH3(CH2)2CO2H | butyric acid | butter (L. butyrum) | butanoic acid | -5.5 ºC | 164 ºC |
| CH3(CH2)3CO2H | valeric acid | valerian root | pentanoic acid | -34.5 ºC | 186 ºC |
| CH3(CH2)4CO2H | caproic acid | goats (L. caper) | hexanoic acid | -4.0 ºC | 205 ºC |
| CH3(CH2)5CO2H | enanthic acid | vines (Gk. oenanthe) | heptanoic acid | -7.5 ºC | 223 ºC |
| CH3(CH2)6CO2H | caprylic acid | goats (L. caper) | octanoic acid | 16.3 ºC | 239 ºC |
| CH3(CH2)7CO2H | pelargonic acid | pelargonium (an herb) | nonanoic acid | 12.0 ºC | 253 ºC |
| CH3(CH2)8CO2H | capric acid | goats (L. caper) | decanoic acid | 31.0 ºC | 219 ºC |
Example (Common Names Are in Red)
Naming carboxyl groups added to a ring
When a carboxyl group is added to a ring, the suffix -carboxylic acid is added to the name of the cyclic compound. The ring carbon attached to the carboxyl group is given the #1 location number.
The acid with the carboxyl group attached directly to a benzene ring is called benzoic acid (C6H5COOH).

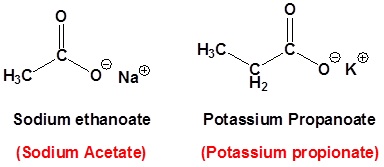
Naming carboxylates
Salts of carboxylic acids are named by writing the name of the cation followed by the name of the carboxylic acid with the –ic acid ending replaced by an –ate ending. This is true for both the IUPAC and Common nomenclature systems.
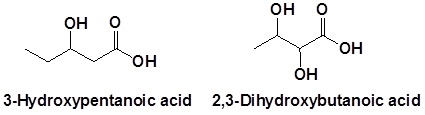
Naming carboxylic acids which contain other functional groups
Carboxylic acids are given the highest nomenclature priority by the IUPAC system. This means that the carboxyl group is given the lowest possible location number and the appropriate nomenclature suffix is included. In the case of molecules containing carboxylic acid and alcohol functional groups, the OH is named as a hydroxyl substituent. However, the l in hydroxyl is generally removed.
In the case of molecules containing a carboxylic acid and aldehydes and/or ketones functional groups, the carbonyl is named as an "Oxo" substituent.
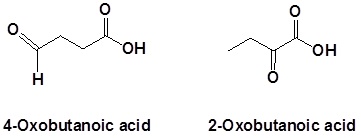
In the case of molecules containing a carboxylic acid an amine functional group, the amine is named as an "amino" substituent.
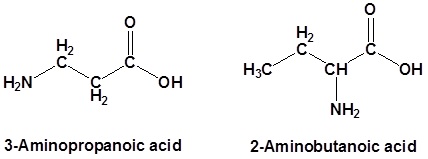
When carboxylic acids are included with an alkene the following order is followed:
(Location number of the alkene)-(Prefix name for the longest carbon chain minus the -ane ending)-(an –enoic acid ending to indicate the presence of an alkene and carboxylic acid)
Remember that the carboxylic acid has priority so it should get the lowest possible location number. Also, remember that cis/tran or E/Z nomenclature for the alkene needs to be included if necessary.
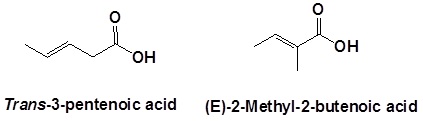
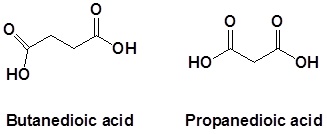
Naming dicarboxylic acids
For dicarboxylic acids the location numbers for both carboxyl groups are omitted because both functional groups are expected to occupy the ends of the parent chain. The ending –dioic acid is added to the end of the parent chain name.
Naming Nitriles, R−C≡N
A nitrile is any organic compound with a −C≡N functional group. In literature the prefix cyano- is used interchangeably with the term nitrile to refer to the functional group. Nitriles used to be known as cyanides; the smallest organic nitrile is ethanenitrile, CH3CN, (old name: methyl cyanide or acetonitrile).
Open chain nitriles are named with the word -nitrile after the name of the parent alkane name. Remmber to include the carbon atom of the nitrile as part of the parent chain. For example, CH3CN has two carbons including the nitrile carbon, therefore it is ethanenitrile. The carbon in the nitrile is given the #1 location position. It is not necessary to include the location number in the name because it is assumed that the functional group will be on the end of the parent chain.
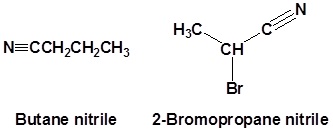
When a nitrile is the highest priority functional group attached to a cycloalkane, the name of the parent cycloalkane is followed by the word -carbonitrile. The ring carbon attached to the nitrile is numbered C1 and the nitrile is not given a number in the name.
Nitriles are often named based off the common name of the corresponding carboxylic acid. This is done by replacing the -ic acid or -oic acid ending with -onitrile. In these cases the nitrile carbon is numbered C1 but the nitrile itself is not given a number in the name.
In the case of molecules containing a carboxylic acid and nitrile functional group, the nitrile is named as a "cyano" substituent. Note! The carbon in the nitrile is not counted as part of the parent chain when named as a cyano substituent.

