20.0: Chapter Objectives and Introduction to Carboxylic Acids
- Page ID
- 36559
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- fulfill all of the detailed objectives listed under each individual section.
- design multi‑step syntheses in which the reactions introduced in this chapter are used in conjunction with any of the reactions described in previous chapters.
- solve road‑map problems which require a knowledge of the chemistry of carboxylic acids.
- define, and use in context, the key terms introduced in this chapter.
The global demand for acetic acid is about 6.5 million tonnes per year. A common industrial preparation of acetic acid is the catalytic oxidation of methanol with carbon monoxide. Much of the acetic acid produced is converted to vinyl acetate polymer and used in adhesives and paints.
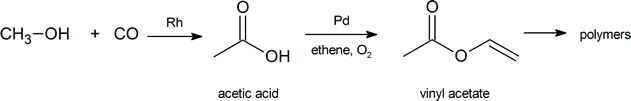
Not only are carboxylic acids valuable, but they and their derivatives are useful starting materials for many synthetic products. Finally, carboxylic acids and their derivatives are also found in a variety of natural systems and important biological pathways.
Carboxylic Acids
Carboxylic acids have been known throughout human history. Prehistoric people likely made acetic acid when their fermentation reactions went awry and produced vinegar instead of wine. The Sumerians (2900–1800 BCE) used vinegar as a condiment, a preservative, an antibiotic, and a detergent. Vinegar contains 4 to 5 percent acetic acid. Acetic acid gives vinegar its sour taste and pungent odor and can do the same thing to wine. Acetic acid, CH3COOH, is an example of the class of compounds called carboxylic acids, each of which contains one or more carboxyl groups, COOH. The general formula of a carboxylic acid is RCOOH.
Citric acid was discovered by an Islamic alchemist, Jabir Ibn Hayyan (also known as Geber), in the 8th century, and crystalline citric acid was first isolated from lemon juice in 1784 by the Swedish chemist Carl Wilhelm Scheele. Medieval scholars in Europe were aware that the crisp, tart flavor of citrus fruits is caused by citric acid.
Citric acid
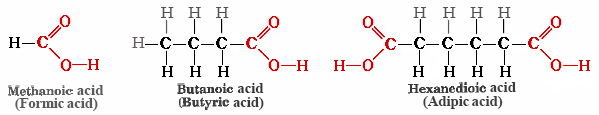
Naturalists of the 17th century knew that the sting of a red ant’s bite was due to an organic acid that the ant injected into the wound. Formic acid (the name comes from Latin word formica meaning “ant“) is present in ants and bees and is responsible for the burning pain of their bites and stings. Butyric acid, a component of rancid butter and Limburger cheese, has a vile odor. Adipic acid is an example of a dicarboxylic acid—it has two functional groups—and is used to make nylon
Naturally Occurring Carboxylic Acids
Carboxylic acids are widespread in nature, often combined with other functional groups. Simple alkyl carboxylic acids, composed of four to ten carbon atoms, are liquids or low melting solids having very unpleasant odors. The fatty acids are important components of the biomolecules known as lipids, especially fats and oils. As shown in the following table, these long-chain carboxylic acids are usually referred to by their common names, which in most cases reflect their sources. A mnemonic phrase for the C10 to C20 natural fatty acids capric, lauric, myristic, palmitic, stearic and arachidic is: "Curly, Larry & Moe Perform Silly Antics" (note that the names of the three stooges are in alphabetical order).
| Formula | Common Name | Melting Point |
|---|---|---|
| CH3(CH2)10CO2H | lauric acid | 45 ºC |
| CH3(CH2)12CO2H | myristic acid | 55 ºC |
| CH3(CH2)14CO2H | palmitic acid | 63 ºC |
| CH3(CH2)16CO2H | stearic acid | 69 ºC |
| CH3(CH2)18CO2H | arachidic acid | 76 ºC |
Interestingly, the molecules of most natural fatty acids have an even number of carbon atoms. Since nature makes these long-chain acids by linking together acetate units, it is not surprising that the carbon atoms composing the natural products are multiples of two. Analogous compounds composed of odd numbers of carbon atoms are perfectly stable and have been made synthetically. All the double bonds in the unsaturated compounds listed on the table have cis (or Z) stereochemistry.
| Formula | Common Name | Melting Point |
|---|---|---|
| CH3(CH2)5CH=CH(CH2)7CO2H | palmitoleic acid | 0 ºC |
| CH3(CH2)7CH=CH(CH2)7CO2H | oleic acid | 13 ºC |
| CH3(CH2)4CH=CHCH2CH=CH(CH2)7CO2H | linoleic acid | -5 ºC |
| CH3CH2CH=CHCH2CH=CHCH2CH=CH(CH2)7CO2H | linolenic acid | -11 ºC |
| CH3(CH2)4(CH=CHCH2)4(CH2)2CO2H | arachidonic acid | -49 ºC |
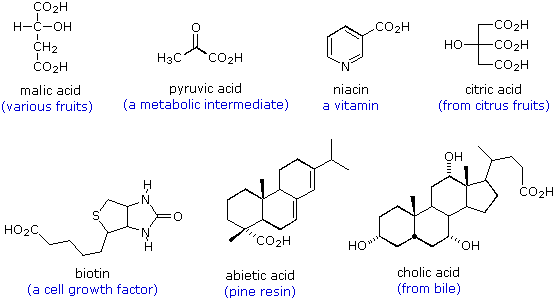
The following formulas are examples of other naturally occurring carboxylic acids. The molecular structures range from simple to complex, often incorporate a variety of other functional groups, and many are chiral.
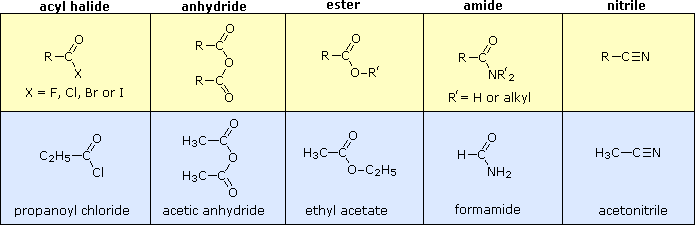
Carboxylic Acid Derivatives
Other functional group combinations with the carbonyl group can be prepared from carboxylic acids, and are usually treated as related derivatives. The chemistry behind the conversion of carboxylic acids to carboxylic acid derivatives will be discussed in then next chapter. Five common classes of these carboxylic acid derivatives are listed in the following table. Although nitriles do not have a carbonyl group, they are included here because the functional carbon atoms all have the same oxidation state. The top row shows the general formula for each class, and the bottom row gives a specific example of each. As in the case of amines, amides are classified as 1º, 2º or 3º, depending on the number of alkyl groups bonded to the nitrogen.