20.4: Substituent Effects on Acidity
- Page ID
- 36553
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- list a given series of carboxylic acids in order of increasing or decreasing acidity.
- explain the difference in acidity between two or more given carboxylic acids.
- arrange a series of substituted benzoic acids in order of increasing or decreasing acidity.
- determine whether a given substituted benzoic acid will be more or less acidic than benzoic acid.
- decide which of two or more substituted benzoic acids is the most acidic, and explain your decision on the basis of the electron‑withdrawing or electron‑releasing ability of the substituent.
- use the Ka (or pKa ) of a substituted benzoic acid to predict the effect that the substituent has on the susceptibility of the benzene ring to electrophilic attack.
You have already seen how the presence of an electron‑withdrawing or electron‑releasing group affects the stability of a positively charged carbocation. Now you see how these groups affect the stability of carboxylate anions, and in turn, determine the dissociation constant of a carboxylic acid.
The Inductive Effect
As seen in Section 20.2, there can be substantial range in the acidities of carboxylic acids. From the table in Section 20.2, we see that trifluoroacetic acid (Ka = 0.59) is almost than 60,000 times more acidic than butanoic acid (Ka = 1.51 X 10-5). These vast differences in acidity can be almost exclusively explained by the inductive effect of substituents attached to the carboxylic acids.
The inductive effect is an experimentally observed effect of the transmission of charge through a chain of atoms in a molecule, resulting in a permanent dipole in a bond. In a carboxylic acid group the presence of halogens (such as fluorine) on adjacent carbons increases the acidity of the carboxylic acid group by stabilizing the carboxylate conjugate base.
Withdrawing Inductive Effects
A fluorine atom is more electronegative than a hydrogen atom, and thus it is able to ‘induce’, or ‘pull’ the electron density of covalent bonds towards itself. In the fluoroacetate anion, the electrons in the C-F covalent bond are pulled toward the fluorine giving the carbon a partial positive charge. The positively charged carbon, in turn, draws electron density away from the carboxylate anion, dispersing the charge, and creating a stabilizing effect. Stabilizing the carboxylate anion increases the acidity of the corresponding carboxylic acid. In this context, the fluorine substituent is acting as an electron-withdrawing group.
Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine
A similar effect is seen when other electron-withdrawing groups are attached to -CH2CO2H. As the power of the electron-withdrawing group becomes stronger there is a corresponding drop in the pKa of the carboxylic acid.
The presence of multiple electron-withdrawing groups compounds the inductive effect and continues to increase the acidity of the carboxylic acid. Dichloroacetic is a stronger acid than chloroacetic acid, and trichloroacetic acid is even stronger.
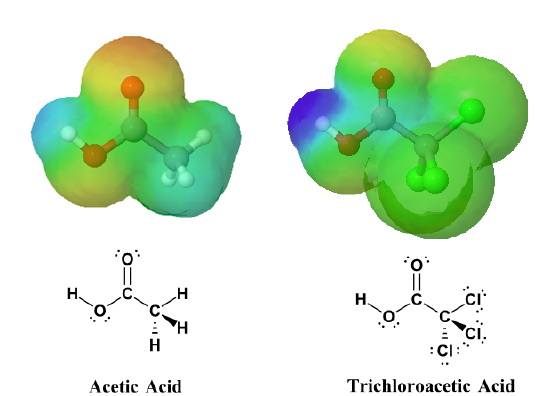
The inductive effects of chlorine be clearly seen when looking at the electrostatic potential maps of acetic acid (Left) and trichloroacetic acid (Right). The O-H bond in trichloroacetic acid is highly polarized, as shown by the dark blue color making it a much stronger acid than acetic acid.
Because inductive effects are not transmitted effectively through covalent bonds, the acid-strengthening effect falls off rapidly as the number of sigma bonds between the carboxylic acid and the electron-withdrawing group increases. A distance of three sigma bonds almost completely eliminates chlorine's inductive effect in 4-chlorobutanoic acid, giving it a similar pKa value to unsubstituted butanoic acid.
Donating Inductive Effects
Alkyl groups (hydrocarbons) are inductively electron-donating. In this case, the inductive effects pushes electron density onto the carboxylate anion, producing a destabilizing effect, decreasing the acidity of the carboxylic acid.
Lengthening the alkyl chain of a carboxylic acid can increase this inductive effect but it no longer decreases the acidity further after the chain is about three carbons long.
The Acidity of Substituted Benzoic Acids
Extensive research has been performed on the acidity of substituted benzoic acids. Benzoic acid itself is a somewhat stronger acid than acetic acid. The carboxyl group of benzoic acid is attached to an sp2-hybridized carbon which is more electronegative and electron-withdrawing than the sp3-hybridized carbon attached to the carboxyl group of acetic acid. In Section 2.9 it was discussed that carbon becomes more electron-withdrawing as the s character of its hybrid orbitals increases. The same effect is seen in acrylic acid which is also more acidic than acetic acid.
In Section 16.4 it was discussed that substituents had a marked effect on the electron density of an aromatic ring and on the rate of electrophilic aromatic substitutions. Electron-donating substituents increase the electron density of the aromatic ring, activating it, and increasing the rate of electrophilic substitutions. Electron-withdrawing groups decreased electron density, deactivating the aromatic ring, and reduced the rate of electrophilic substitution.
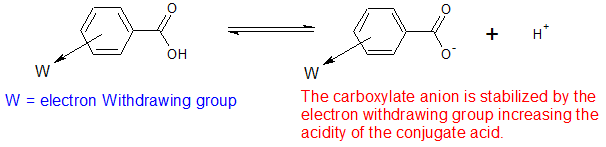
In a similar fashion, substituents have an effect on the acidity of benzoic acids. Benzoic acids with deactivating substituents, such a nitro group (-NO2), in the meta or para position tend to be more acidic. The deactivating substituents remove electron density, stabilizes the negative charge of the carboxylate anion, and increasing the acidity of the carboxylic acid.
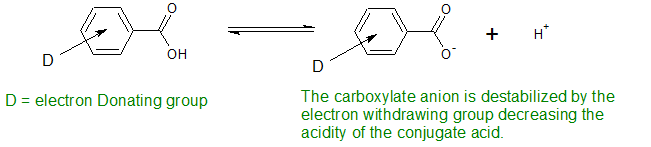
The opposite effect occurs with electron donating substituents. Electron-donating substituents in the meta or para position destabilize the carboxylate anion making the benzoic acid less acidic.
A closer look at the effects of electron-withdrawing and electron-donating substituents in the meta or para position can be seen in the pKa values of various benzoic acids as shown in the table below. Notice the trend in the following table where electron donating substituents (X) at the meta or para positions lead to weaker acids while those having more electron withdrawing substituents generate stronger acids.
| X | pKa-Para | pKa-Meta | Substituent Type | |
|---|---|---|---|---|
| —NH2 | 4.8 | 4.7 | Donating |  |
| —OH | 4.6 | 4.1 | Donating | |
| —OCH3 | 4.50 | 4.1 | Donating | |
| —CH3 | 4.4 | 4.3 | Donating | |
| —H | 4.20 | 4.20 | ||
| —Cl | 4.00 | 3.8 | Withdrawing | |
| —Br | 4.0 | 3.8 | Withdrawing | |
| —NO2 | 3.4 | 3.5 | Withdrawing |
| X | pKa-Ortho | Substituent Type | |
|---|---|---|---|
| —NH2 | 4.7 | Donating |  |
| —H | 4.20 | ||
| —OCH3 | 4.1 | Donating | |
| —CH3 | 3.9 | Donating | |
| —OH | 3.0 | Donating | |
| —Cl | 2.9 | Withdrawing | |
| —Br | 2.9 | Withdrawing | |
| —NO2 | 2.2 | Withdrawing |
Example
The following molecule, p-cyanobenzoic acid, has a pKa of 3.55. Does the cyano substituent activate or deactivate the aromatic ring towards electrophilic aromatic substitution?
The pKa of benzoic acid is 4.2 which means it is a weaker acid than p-cyanobenzoic acid. This this means that the cyano substituent is deactivating the ring.
1. Draw the bond-line structures and arrange the following compounds in order of increasing acidity: 4-nitrobenzoic acid; 4-aminobenzoic acid; 4-chlorobenzoic acid; and benzoic acid. Try to use the expected inductive effects of the substituents to determine the acidity rather than looking at the pKa table.
- Answer
-
1.
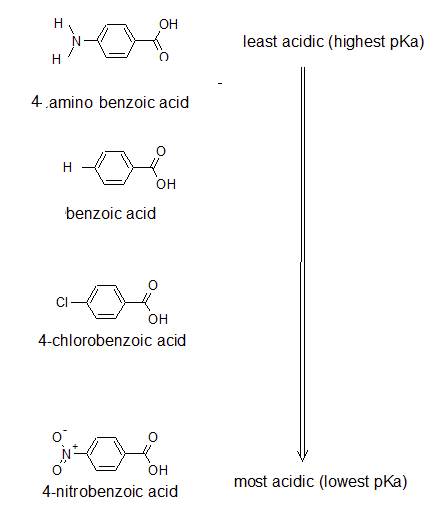
Exercises
1) For the following pairs, which is expected to be the stronger acid? Explain your answer.
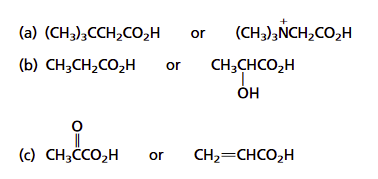
d) acrylic aid vs. propiolic acid (image)
2) Oxalic acid is a dicarboxylic acid with two acidic protons. The first proton is much more acidic (pKa = 1.20) than a typical carboxylic acid. However, Heptanedioic acid's first acidic proton has a pKa much closer to that of a typical carboxylic acid. Explain these differences.
(image)
3) The carboxylic acid of 4-formylbenzoic acid has a pKa of 3.75. Is this molecule likely to be more reactive or less reactive than benzene toward electrophilic aromatic substitution?
Solutions
1)
a) Consider the inductive effects of the substituents attached to the carboxylic acid. The tert-butyl group is electron-donating which should decrease the acidity of the carboxylic acid. The trimethylammonium substituent is positively charged and can be a powerful electron-withdrawing substituent. This should increase the acidity of the carboxylic acid. The compound (CH3)3NCH2CO2H is expected to be a stronger acid than (CH3)3CCH2CO2H. The acidity constants for these two compounds match the predictions.
(image)
b) Having an electron-withdrawing hydroxyl group at the C-2 stabilizes the carboxylate ion of lactic acid through inductive effects.
(image)
This should make lactic acid more acidic than propanoic acid.
c) Due to the presence of a highly electronegative oxygen, the carbonyl group is expected to be more strongly electron-withdrawing than a carbon–carbon double bond. Thus, pyruvic acid should be a stronger acid than acrylic acid.
(image pyruvic and acrylic acid)
d) The main difference between the two compounds is the hybridization of the carbon attached to the carboxylic acid. The alkyne substituent has an sp hybridized carbon which should make it more electron-withdrawing than the alkene's sp2 hybridized carbon.
2) With oxalic acid one carboxyl group acts as an inductive electron-withdrawing group which increases the acidity of the other carboxylic acid. This inductive effect is only relevant with the two carboxyl groups are separated by only a few bonds. In heptanedioic acid, the carboxyl groups are separated by five carbon which effectively negates the inductive effect.
3) Benzoic acid (pKa = 4.2) has a higher pKa and is more acidic than 4-formylbenzoic acid (pKa = 3.75). This means that the formyl group is removing electrons from the aromatic ring making it deactivated toward electrophilic aromatic substitution.

