9: A return to the carbonyl
- Page ID
- 353909
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)As we have seen carbonyl compounds undergo both acid and base catalyzed reactions involving nucleophilic attack at the carbonyl carbon (or at the beta carbon of conjugated carbonyls). This reaction, in its many guises, can produce an impressive range of products from the formation of an ester from an acid to the production of alcohols from carbonyls. While these reactions may seem superficially different, if you understand the underlying mechanism involved, it is possible to make plausible predictions for the outcome for literally thousands of reactions. By this time, you should have come to understand such processes. Now, it is time to reconsider carbonyl groups in light of the fact that there is a completely different set of reactions that involve the reactivity of the alpha carbon of the carbonyl groups. Carbonyl compounds typically exist in two tautomeric forms: the keto and enol forms. The keto form is usually the major tautomer and there is always some enol present as well.
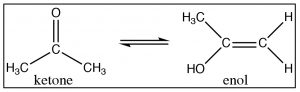
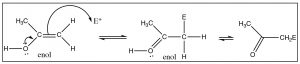
The structure of the enol form can provide clues about its different reactivity, which is distinct from that of the keto form. The enol form consists of an alcohol directly attached to a \(\mathrm{C}\) that is involved in a double bond. As we know, alkenes are electron-rich and tend to undergo electrophilic attack; the presence of an attached \(\mathrm{-OH}\) group makes such an electrophilic attack even more likely. Just like an \(\mathrm{-OH}\) group on an aromatic ring, the \(\mathrm{OH}\) can donate electrons through resonance with the \(\mathrm{-C=C-}\) and make the enol more reactive. Carbonyl groups can react, through the small percentage of the enol form present, to undergo electrophilic attack at the alpha carbon.
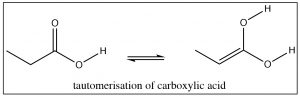
We have already seen that aldehydes and ketones exist as keto-enol tautomers, but, in fact, carboxylic acids, esters, and other acid derivatives also have the potential to exist in the corresponding enol form.
Another implication of the alcohol-nature of an enol is that we expect it to be acidic—and indeed it is. The conjugate base of the enol is called the enolate ion and it is resonance-stabilized so that the negative charge is delocalized on both the oxygen and on the alpha carbon.
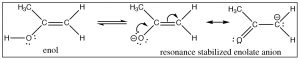
The \(\mathrm{pK}_{\mathrm{a}}\) of acetone is \(19\)—somewhat higher than a typical alcohol (\(\mathrm{pK}_{\mathrm{a}} \sim 15\)). In the enolate form, the majority of the charge sits on the more electronegative oxygen, but a significant proportion of the negative charge is associated with the alpha carbon: the enolate ion is a stabilized carbanion. The enolate anion is often written in its carbanion form because this is the form that produces most of the interesting chemistry. Treatment of a carbonyl compound with a base, such as an alkoxide, results in the reversible formation of a small amount of the enolate ion (although the equilibrium lies on the side of the unprotonated form). Similarly, many carbonyl compounds can be deprotonated to give the corresponding enolate anion. For example, esters can be treated with a base to give the corresponding enolate anion.
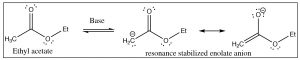
Ethyl acetate has a \(\mathrm{pK}_{\mathrm{a}}\) of around \(25\) (less acidic than acetone: \(\mathrm{pK}_{\mathrm{a }} 19\)), but still well within reach of many of the strong bases. For example, sodium amide (\(\mathrm{NaNH}_{2}\)), the conjugate base of ammonia (\(\mathrm{pK}_{\mathrm{a }} 33\)), is strong enough to deprotonate the ester. In fact, we typically use what is known as a hindered base, such as lithium di-isopropylamide (\(\mathrm{LDA}\)), which is similar to sodium amide but the nitrogen has two bulky isopropyl groups attached to it.[1]
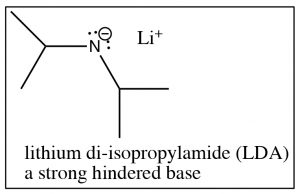
Since \(\mathrm{LDA}\) is such a strong base, treatment of most carbonyl compounds with \(\mathrm{LDA}\) produces essentially 100% of the corresponding enolate anion. However, there are exceptions. Any carbonyl compound that has a more acidic proton than the \(\mathrm{H}\) associated with the alpha carbon will not undergo this reaction. For example, treatment of carboxylic acids with \(\mathrm{LDA}\) will merely result in the loss of the acidic proton from the carboxylic acid OH group.
Most carbonyl compounds have \(\mathrm{pK}_{\mathrm{a}}\)‘s between \(19\) and \(25\). Compounds that have carbonyl groups that are beta to each other (that is, separated by an intervening carbon), have significantly lower \(\mathrm{pK}_{\mathrm{a}}\)‘s (around \(9\)), because the resulting anion can be stabilized on both carbonyl oxygens.
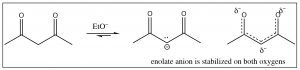
They can be easily deprotonated by bases such as sodium ethoxide or sodium hydroxide.


