9.8: Glycolysis- From Glucose to Fructose
- Page ID
- 355072
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
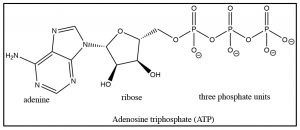
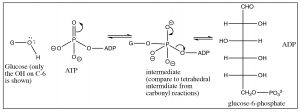
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The first steps of the biological cycle known as glycolysis involve the conversion of glucose to fructose. As we will see, this sets up the reverse aldol reaction that results in the splitting of glucose into two 3-carbon fragments—but we will get to that shortly. The first step in glycolysis is the phosphorylation of the \(\mathrm{OH}\) group at \(\mathrm{C-} 6\). This reaction is analogous to the formation of a carboxylate ester, the only difference being that the attack occurs at a phosphorus rather than a carbon. The source of phosphate is adenosine triphosphate (\(\mathrm{ATP}\)) and, in this case, it is the terminal phosphate that is transferred to the glucose and the leaving group is \(\mathrm{ADP}\) (adenosine diphosphate). The reaction is mediated by an enzyme (a kinase): this reaction is highly thermodynamically favorable (\(\Delta \mathrm{G}\) is negative).
The consequences of glucose phosphorylation reaction are twofold: First, it serves to keep the concentration of glucose in the cell low, so that glucose can continuously diffuse into the cell (through membrane channel proteins). Glucose-6-phosphate is highly charged (the oxygens on the phosphate group are almost entirely ionized at physiological \(\mathrm{pH}\)) and cannot diffuse back out of the cell (through the channel). The second consequence is that the hydroxyl group on \(\mathrm{C-} 6\) has been activated. The phosphate is an excellent leaving group.
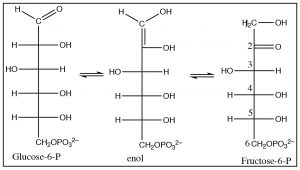
The next step is the transformation of glucose into fructose. In organic chemistry terms, this is a simple tautomerization as fructose is a structural isomer of glucose. The enzyme that regulates this conversion is known as an isomerase.
The glucose aldehyde undergoes enolization as shown, followed by another tautomerization to produce the fructose ketone. All of these reactions are entirely analogous to the keto-enol interconversions that we have seen in simpler systems.
The next step is another phosphorylation reaction at \(\mathrm{C-} 1\) to produce fructose-1,6-diphosphate; it involves the same reaction mechanism that produced phosphorylation at \(\mathrm{C-} 6\) using \(\mathrm{ATP}\) as the source of phosphate. You might be asking, how is it that these reactions occur in this particular sequence and why don’t the other oxygens undergo phosphorylation? The answer to this lies in the fact that these reactions are mediated by enzymes that guide the substrates into the correct (pre-established, evolutionarily) orientations. Reactions in solution depend entirely on random collisions with enough energy to surmount the activation energy barrier and in the correct orientation. In biological systems, the substrate first collides with, and its orientation is constrained through interactions with, the enzyme; limiting subsequent aspects (steps) of the reaction. While there is potential for other hydroxyls to be phosphorylated (and it would certainly be difficult to control the site of attack if the molecules were free in solution, the reactant-enzyme complex favors certain ones dramatically over others. Remember the enzyme itself is result of evolutionary mechanisms. Given the ubiquity of this process, it was likely established early, and present in the last common ancestor of life.
The Reverse Aldol:
The system is now set up for the cleavage of the six-carbon sugar into two three-carbon species via a reverse aldol reaction as shown.
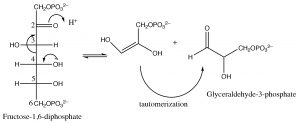
The result is the production of two molecules of glyceraldehyde-3-phosphate from one molecule of glucose. Up to this stage, glycolysis has involved the use of two \(\mathrm{ATP}\) molecules associated with the two phosphorylation reactions.
In the next stage of glycolysis, another phosphate is added to each glyceraldehyde-3-phosphate molecule; this phosphate is not derived from \(\mathrm{ATP}\), but from inorganic phosphate. The mechanism involves an addition of a phosphate unit to the aldehyde carbonyl, followed by oxidation as the tetrahedral intermediate collapses, with loss of the hydride ion that adds to \(\mathrm{NAD}^{+}\), to form \(\mathrm{NADH}\).
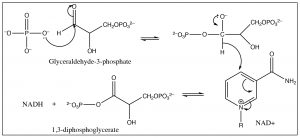
The resultant species now contains two phosphate groups, but they are different chemically: the phosphate attached to the newly oxidized carbon is much more reactive than the other. Attack by a nucleophile at the phosphorus preferentially expels a carboxylate anion from the intermediate, rather than an alkoxide. In the next step of the reaction, the highly reactive phosphate is transferred to \(\mathrm{ADP}\) through an attack by the \(\mathrm{O}^{-}\) on the terminal phosphate of \(\mathrm{ADP}\) onto the \(\mathrm{P}\) of the 1,3-diphosophoglycerate, with subsequent loss of 3-phosphoglycerate (because the carboxylate is a good leaving group).
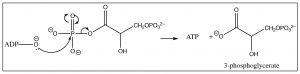
This reaction produces two \(\mathrm{ATP}\) molecules (and 2 reduced \(\mathrm{NADH}\) molecules) from one original glucose (because there are now two three-carbon units), so, at this stage, the net production of \(\mathrm{ATP } = 0\). The transfer of the other phosphate unit from \(\mathrm{C-} 3\) to another \(\mathrm{ADP}\) does not occur because the leaving group (an alkoxide) is not thermodynamically favorable. Instead, the phosphate group is transferred from \(\mathrm{C-} 3\) to \(\mathrm{C-} 2\) by an intermolecular nucleophilic attack that forms a five-membered ring intermediate and subsequent elimination of water.
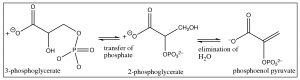
The product of this elimination reaction is called phosphoenol pyruvate (PEP). In essence, it is an enol that has been trapped by esterification with the phosphate. The enol is an excellent leaving group (since it is really a carbonyl group) and, therefore, PEP will also undergo attack by \(\mathrm{ADP}\) to produce \(\mathrm{ATP}\) and pyruvate, resulting in a in a net \(+ 2 \mathrm{ ATP}\) molecules for the overall glucose to pyruvate reaction (plus the two \(\mathrm{NADH}\) molecules).
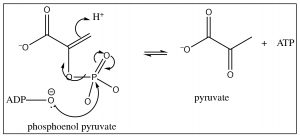
Once again, our intent here is not to have you memorize this long sequence of reactions, but rather to recognize that even in systems that appear highly complex, when each reaction is viewed at the molecular level, it is recognizable as the same reactions that we have been studying. In fact, the mechanisms of a large majority of reactions in biological systems can be understood in relatively simple terms. Many of the reactions are the same: attack at carbonyl (or phosphate) groups, aldol and retro aldol condensations, and keto-enol tautomerizations.

