9.7: Glycolysis
- Page ID
- 354680
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Glycolysis is the name we give to the group of reactions that result in the splitting of a \(\mathrm{C-} 6\) glucose molecule into two \(\mathrm{C-} 3\) units, which is accompanied by the overall production of \(\mathrm{ATP}\), the molecule that can be used to provide energy to drive unfavorable chemical reactions such as building up biopolymers like peptides, nucleic acid polymers (\(\mathrm{DNA}\) and \(\mathrm{RNA}\)), and production of fats. This process is usually depicted schematically, particularly in biology texts, but every step of the process is a relatively simple organic reaction that can be understood in terms of the principles that we have learned over the past year. The overall schematic for glycolysis is given below[2], but our intent here is not that you memorize each step so that it can be regurgitated; it’s to allow you to understand how and why these reactions occur the way that they do (or at least the way they do in biological systems).
We will be treating these reactions from an organic chemistry perspective, but it is important to note that in the body all of these reactions are mediated by enzymes and co-factors that lower the activation energy for each reaction. The first part of the glycolysis pathway involves the conversion of the sugar glucose, to a different sugar, fructose. Therefore we will begin by looking briefly at the structure and properties of sugars.
Glucose:
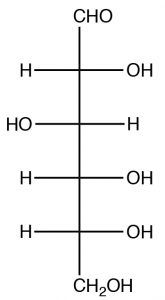
While glucose looks complex, we have already investigated the functional groups present and all the reactions that are important here. Glucose belongs to the family of compounds called sugars part of a larger group, known as carbohydrates, denoted by the suffix -ose. Since it has six carbons, glucose is known as hexose (similarly, a five-carbon sugar would be known as a pentose). Glucose can exist in several forms; both open and closed chain. We consider the open-chained form first. The easiest way to represent sugars is by using a Fischer projection (in fact these representations were invented for just this purpose). Remember that in a Fischer projection, the horizontal bonds are pointing out of the plane and are all eclipsed. The naturally occurring form of glucose is D-Glucose \(\rightarrow\).
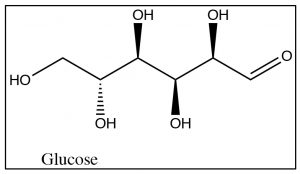
Note that there are four chiral centers in glucose, and therefore there are \(16\) (\(2^{4}\)) possible stereoisomers, many of which do occur naturally. The D designation has to do with the stereochemistry at position 5 and does not refer to the direction of the rotation of plane-polarized light. (While it is possible to designate R or S for each chiral center[3], it is not possible to designate R or S for the molecule as a whole). Note that glucose also has an aldehyde group (at position 1) and, therefore, also belongs to the class of sugars called aldoses.
In its open chain form, glucose has an aldehyde group and five hydroxyl groups and, as you might expect, there is great potential here for reactions, both inter- and intra-molecular. In a solution, glucose commonly exists in the hemiacetal form, in which the \(\mathrm{OH}\) group on \(\mathrm{C-} 5\) has attacked the carbonyl group to form a six-membered ring which is referred to as the pyranose form (pyran is a six-membered heterocyclic ring with an oxygen atom in it). The pyranose form is usually drawn in the chair form as shown below.
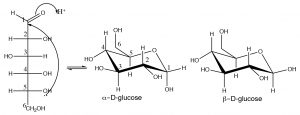
Hemiacetal formation produces two possible configurations, but rather than calling them R and S, we label them alpha and beta. In alpha form, the \(\mathrm{OH}\) on carbon 1 is on the opposite side of the ring from the \(\mathrm{CH}_{2}\mathrm{OH}\) (\(\mathrm{C-} 6\) of the original chain), the beta form has the \(\mathrm{OH}\) group on the same side as the \(\mathrm{CH}_{2}\mathrm{OH}\). These two forms are stereoisomers because they have the opposite configuration at \(\mathrm{C-} 1\), but unlike typical stereoisomers, they can be interconverted by ring-opening of the hemiacetal and reclosure of the ring. They are called anomeric forms and \(\mathrm{C-} 1\) is referred to as the anomeric carbon. Such carbons can be identified by the fact that they have two oxygens attached to them. In an aqueous equilibrium solution of glucose, less than 1% is present in the open chain form, but since there is always an equilibrium concentration present, the alpha and beta forms can and will interconvert via this open-chain form. As might be expected, the beta form is more stable (because the \(\mathrm{OH}\) is equatorial) and at equilibrium there is about 64% beta. For sugars such as glucose, there is also the possibility that a five-membered ring can form by the reaction of the alcohol at \(\mathrm{C-} 4\) with the carbonyl. Again, two forms (alpha and beta) are possible, which can be interconverted via ring-opening. This five-membered ring form is called the furanose form (furan is a five-membered ring with one oxygen).
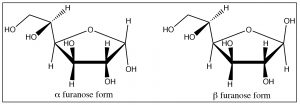
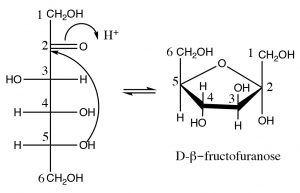
Fructose:
Fructose is another six-carbon sugar. It differs from glucose in that it that has a ketone rather than an aldehyde at \(\mathrm{C-} 2\); for this reason, it is called a ketose (rather than an aldose). Fructose usually exists in a five-membered hemiacetal ring formed by reaction of the \(\mathrm{OH}\) at \(\mathrm{C-} 5\) with the ketone carbonyl at \(\mathrm{C-} 2\). The resulting five-membered ring is called the furanose form (furan is a five-membered ring with one oxygen) and typically furanose rings are depicted using yet another structural representation: the Haworth projection, shown here. In this structure, the ring is drawn as a plane (although it isn’t, of course), and the substituents are either above or below the plane of the ring. In the same way as glucose, fructose can exist in either the alpha or beta forms.