8.12: Nucleophilic Substitutions on Aromatic Systems- Expanding the range of potential substitution products
- Page ID
- 359146
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)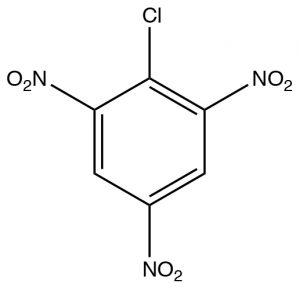
All of the reactions on the aromatic ring so far have proceeded by one mechanism: electrophilic aromatic substitution. Up to now, we have not solved the problem of introducing substituents that usually react as nucleophiles onto an aromatic ring. There are a number of ways to accomplish this and we will consider three of these mechanisms.
The first, Nucleophilic Aromatic Substitution (\(\mathrm{SNAr}\)), is somewhat analogous to an \(\mathrm{S}_{\mathrm{N}} 2\) reaction. It will occur if two conditions are met: the first is there must be a leaving group present on the ring, typically a halide; the second is that that the electron density of the ring must be reduced. This is typically accomplished by having electron-withdrawing groups such as nitro groups on the ring. We can observe the effect of adding electron-withdrawing groups by looking at the \(\mathrm{NMR}\) spectra of 2-chloro-1,3,5-trinitrobenzene. The only peak in the \(\mathrm{H-NMR}\) spectrum is a \({}^{2}\mathrm{H}\) singlet at \(9.1 \mathrm{~ppm}\). This is considerably downfield of the \(7.3 \mathrm{~ppm}\) of benzene because the of the effect of the electron withdrawing nitro groups. This effect explains why the ring is susceptible to nucleophilic attack.
The reaction proceeds by an initial attack by a nucleophile such as \(\mathrm{OH}\), \(\mathrm{OR}\), followed by loss of the leaving group which takes the pair of electrons with it.
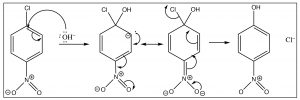
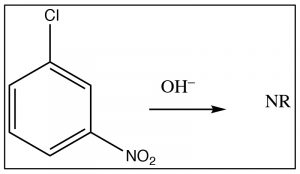
The intermediate anion can be directly stabilized by the nitro group, but only if that group is in the ortho or para position. If the nitro group is meta to the leaving group the reaction will not occur, because such stabilization is not possible (try to draw resonance forms for it).
A second mechanism involves elimination of \(\mathrm{HX}\) from the ring, followed by a rapid addition of \(\mathrm{HY}\); it can be considered analogous to elimination and addition reactions of alkenes and alkynes. The first step is the elimination of \(\mathrm{HX}\) from benzene, which produces a highly reactive, strained species which is called a benzyne. This intermediate undergoes very rapid reaction to add \(\mathrm{H}^{+}\) and \(\mathrm{Y}^{–}\) across the double bond.
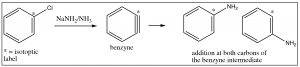
Evidence for this mechanism, rather than \(\mathrm{SNAr}\), comes from isotope labeling studies. If the original site is isotopically labeled (e.g. with \(\mathrm{C}-13\), the final product has only 50% of the nucleophile at the labeled site and 50% at the adjacent carbon. If this were a straight nucleophilic substitution, all of the substitution would take place at the labeled carbon.
Diazonium ions:
Another approach that allows access to multiple products involves the reaction of aniline (\(\mathrm{PhNH}_{2}\)) with the nitronium ion (produced in the reaction mixture) to what is known as a diazonium ion.
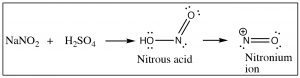
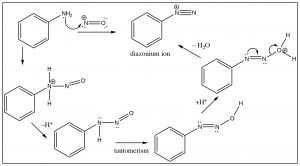
The diazonium ion is highly unstable: it must be prepared at temperatures around \(0^{\circ}\mathrm{C}\); if the solution is warmed above this temperature, it will decompose by losing a molecule of nitrogen (\(\mathrm{N}_{2}\)) to produce a carbocation. It is this decomposition that can be captured by a range of nucleophiles. In some ways this reaction is akin to an \(\mathrm{S}_{\mathrm{N}} 1\) reaction in which a carbocation is produced, which then undergoes rapid nucleophilic attack.
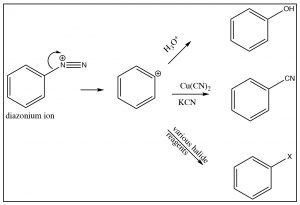
The intermediate carbocation can be captured in the presence of a nucleophile: for example water, alcohol, halide, or cyanide ions. Note that all these reactions typically require a raised temperature or the presence of a metal ion catalyst. This is to enhance the rate of decomposition of the diazonium ion, so that the resulting carbocation can be captured by the nucleophile. It is important to note that this carbocation is NOT resonance-stabilized (you cannot draw resonance forms to stabilize—try it).
Diazo coupling reactions:
Besides being a way to introduce some nucleophiles into aromatic rings, diazonium salts also undergo what is known as a coupling reaction. The diazonium ion itself is susceptible to nucleophilic attack if it is not decomposed too rapidly—usually by another aromatic system. This reaction has been used to produce a wide range of dyes and indicators.
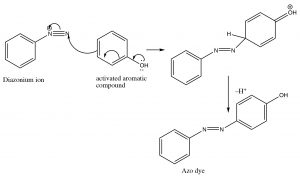
These azo compounds have a long-conjugated chromophore that typically results in absorption of visible light, making the compounds highly colored (as a result of the light that is reflected and not absorbed). Manipulation of groups on either benzene ring can extend the conjugation even further. About 50% of dyes belong to this family of compounds.

