8.11: Multiple Substituents- Directing Effects
- Page ID
- 359145
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)\[An image of disubstituted benzenes.\]
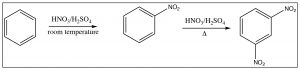
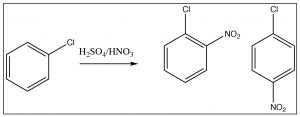
As we have seen, nitrobenzene can be produced by treating benzene with a mixture of nitric and sulfuric acids. If this reaction is carried out at room temperature, nitrobenzene is produced. If the reaction mixture is heated, eventually another product is produced. While there are three possible products (1,2- or 1,3- or 1,4-dinitrobenzene) only 1,3-dinitrobenzene (or meta-nitrobenzene) is formed. In addition, it is formed much more slowly and it is quite easy to isolate only the mono-nitro product.
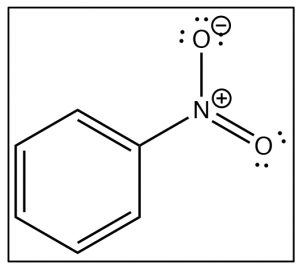
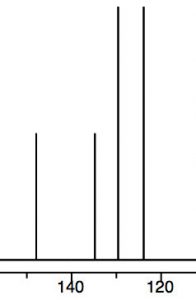
To understand why only one further product is formed and why the rate of the second substitution is slower (so that it requires more energy to surmount the activation energy barrier), let’s take a look at nitrobenzene itself. Notice that the positively charged nitrogen is adjacent to the ring. We might expect that this would remove some electron density from the ring and indeed it does.We can see this effect both in the \(\mathrm{C-}\)13 and \({}^{1}\mathrm{H}\) NMR spectra, where the peaks are shifted to the lower field. Recall that benzene \(\mathrm{H}\) signals all appear at \(7.3\) while in nitrobenzene we see that not only are there different signals, but that they are all at a lower field than benzene. There are chemically distinguishable hydrogens in nitrobenzene (2 ortho, 2 meta and 1 para).
The lowest field \({}^{2}\mathrm{H}\) doublet at \(8.2 \mathrm{~ppm}\) corresponds to the ortho \(\mathrm{H}\)s, the \({}^{2}\mathrm{H}\) signal at \(7.6 \mathrm{~ppm}\) is the two meta \(\mathrm{H}\)s, and the signal at \(7.5\) is the para \(\mathrm{H}\). In the \(\mathrm{C}-13\) NMR spectrum, all the carbons in benzene appear at \(128 \mathrm{~ppm}\). In nitrobenzene, \(\mathrm{C}-1\), the carbon bonded to the nitro group is, as we might expect, shifted downfield to \(148 \mathrm{~ppm}\) because of its proximity to the positively charged nitrogen.

The evidence shows us that nitrobenzene is less electron-rich than benzene—in fact, it reacts with electrophiles 10,000 times more slowly than benzene—but why does it only produce one product on dinitration? The answer to this lies in the structure of the intermediate sigma complex. If you draw out the resonance forms for reaction at the ortho, meta, and para positions, what becomes clear is that the meta position is the only one that doesnot (cannot) place the negative charge on the original nitro-substituted carbon. Both ortho and para substitution are highly disfavored because these intermediates are of such high energy (because of the adjacent positive charges), and, therefore, only the meta product is formed.
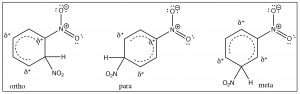
Nitro groups belong to the class of substituents that are deactivating meta directors. Other substitutents that belong in this group are sulfonic acids (\(\mathrm{SO}_{3}\mathrm{H}\)) and any group with a carbonyl next to the ring (aldehydes, ketones, carboxylic acids, and derivatives). All of these groups are destabilized by having a positive charge on the adjacent carbon.
It makes sense then that there is another group of substituents that are activating ortho, para directors. For example, all alkyl groups fall into this classification because they are electron donating by induction. Toluene (methyl benzene) is the simplest example, and indeed when we nitrate toluene, we see a mixture of ortho and para products because now these are the intermediate sigma complexes that can be directly stabilized by induction from the methyl group.
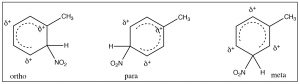
In this case, the ortho and para substitution products are stabilized and produced preferentially. The ratios of products are approximately 2:1 ortho to para (because there are two possible ortho positions). Indeed toluene can be nitrated further until all the ortho and para positions are substituted to give 2,4,6-trinitrotoluene, otherwise known as TNT.[8]

Another group of ortho, para directors are substituents that are attached to the ring by an \(\mathrm{O}\) or \(\mathrm{N}\) (that is, phenol) alkoxybenzenes or aniline and its derivatives. While it might seem, at first glance, that these electronegative atoms would be electron-withdrawing by induction, we know that they can also donate electrons through resonance with the ring. We can see both modes in operation if we look at the evidence from the \(\mathrm{C}-13\) NMR. \(\mathrm{C}-1\) appears at a much lower field (\(158 \mathrm{~ppm}\)) than benzene (\(128 \mathrm{~ppm}\)), presumably because it is directly attached to the electron-withdrawing \(\mathrm{O}\). But the ortho and para carbons appear at a slightly higher field than benzene because these positions are enriched in electron density by resonance.
In fact, phenol is more reactive than benzene and it is difficult to stop the reaction at the mono-nitration step. Again, we observe a mixture of ortho and para products, because the transition state on the way to the intermediate sigma complex can be stabilized by resonance.

Generally, any benzene derivatives with a lone pair that is available for stabilizing the intermediate will be an ortho, para director including aniline (and any N-alkylated analogs) and anisole (methoxybenzene). This also includes compounds that have a lone pair that must be shared between the ring and a carbonyl group: for example, phenyl esters or amides.

There is one more classification for directing groups: the halogens are anomalous in that they are deactivating ortho, para directors. Halogens are electronegative and withdraw electrons from the ring by induction, thus reducing the reactivity. However, halogens also have lone pairs that can be used to stabilize positive charge by resonance. The resonance effect is not as large as it is for \(\mathrm{N}-\) or \(\mathrm{O}-\)substituted rings, but it can still operate. Therefore halo-substituted benzene rings tend to react more slowly than benzene, but they do produce ortho and para products.
Multiple substituents: While it is relatively easy to predict the position of substitution on a monosubstituted ring (one you know how to do), predicting the outcome when there are two or more often becomes problematic. Sometimes, both substituents will “point” to the same positions. For example, in p-nitrophenol, both the \(\mathrm{OH}\) and \(\mathrm{NO}_{2}\) direct the substituent to the same position, but in m-nitrophenol, they direct to different positions as shown.
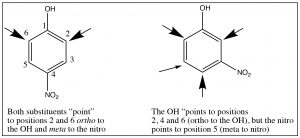
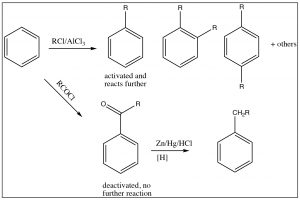
It is, therefore, sometimes possible to predict the position of the third substituent, but, often, it is not. Regardless of this problem, most aromatic substitution reactions have the potential for producing multiple products, either because both ortho and para products are formed or because the product may be more reactive than the reactant. For example, any electrophilic aromatic substitution that adds an activating group to the ring may be difficult to stop at one substitution. Friedel Crafts alkylation is difficult to stop at only one alkylation. In fact, rather than alkylate the ring, it is often preferable to acylate the ring, followed by a reduction of the acyl group. There are a number of reducing reagents that can take the aldehyde or ketone right down to an alkyl group: for example, as shown here, the Clemmensen reduction involves a zinc-mercury amalgam in \(\mathrm{HCl}\).