8.10: Reactions of Aromatic Compounds- Introduction of one group onto the ring
- Page ID
- 359144
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)As we have seen, aromatic compounds are considerably more stable than one might predict. Consequently, it takes more energy to make aromatic compounds undergo reactions since, in order to react the aromatic overlap of orbitals in the ring, it must be destroyed at some point during the reaction. Since the aromatic ring is so electron-rich we might predict that it would undergo electrophilic attack but, rather than undergoing electrophilic addition like alkenes and conjugated alkenes do, aromatic compounds typically undergo electrophilic substitution. For example, benzene will react with bromine in the presence of a catalyst such as iron (III) bromide (FeBr3) to give bromobenzene.

Let us now take a closer look at the steps involved in this reaction to see how this substitution (\(\mathrm{Br}\) for \(\mathrm{H}\)ivc) is accomplished. Since benzene is so stable, a more reactive electrophile is needed to react with the ring. This is accomplished by adding a Lewis acid catalyst \(\mathrm{FeBr}_{3}\), which forms a complex with the bromine to produce \(\mathrm{Br}^{+}\) (stabilized by the \(\mathrm{FeBr}_{4} {}^{-}\)). The \(\mathrm{Br}^{+}\) electrophile now reacts with the electron-rich benzene ring to produce a resonance-stabilized intermediate called a sigma complex.
![]()
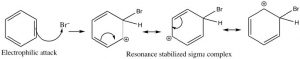
Now, instead of a nucleophile attacking, the aromatic ring is regenerated by loss of a proton.

The resulting bromobenzene is much more stable than the corresponding addition product.
 c
c
This electrophilic substitution reaction is the primary mechanism by which most aromatic compounds react. A very reactive electrophile must be generated; it then adds to one of the ring carbons followed by loss of a proton from the same carbon.
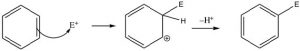
There are a number of substituents that can be introduced onto the ring in this way, including nitro, alkyl, acyl, and sulfonyl groups. Each proceeds via a similar mechanism (via a reactive electrophile that is generated in the reaction) either by using a catalyst or by using very reactive reagents.
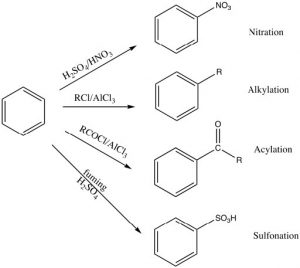

Alkylation and acylation can be accomplished by treatment of an alkyl halide or acyl halide with a Lewis acid catalyst such as aluminum trichloride (\(\mathrm{AlCl}_{3}\)). In this case, the reactive electrophile is generated when the alkyl (or acyl) halide interacts with the catalyst to produce an intermediate that is carbocation-like. It is this very reactive species that reacts with the benzene ring to produce the substituted benzene. This reaction is called a Friedel-Crafts alkylation (or acylation). The acylation reaction is usually preferred because it is difficult to stop an alkylation reaction at just one substitution since the product is more reactive than the starting material (see below).
Nitration is accomplished by treating benzene with a mixture of concentrated nitric and sulfuric acids. This mixture generates a nitronium ion (\(\mathrm{NO}_{2} {}^{+}\)), which is the reactive electrophile.

Sulfonation is accomplished by using fuming sulfuric acid, which actually contains sulfur trioxide (\(\mathrm{SO}_{3}\)) dissolved in the sulfuric acid. It is the actually the \(\mathrm{SO}_{3}\) that is the electrophile in this case.
Some groups cannot be introduced directly onto the ring: for example, groups that we might normally consider as nucleophiles (such as \(\mathrm{NH}_{2}\) or \(\mathrm{OH}\)) have to be introduced indirectly. For example, aniline (aminobenzene) can be produced from nitrobenzene by reduction.
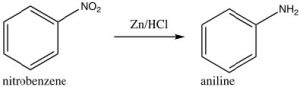
As we will see later, other nucleophilic groups have to be introduced by a different approach.

