8: Conjugated compounds and aromaticity
- Page ID
- 353908
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Conjugation: Conjugation is the term we use to describe an arrangement of alternating single and double bonds. To explain how conjugated systems behave differently from non-conjugated systems we will compare 1,3-pentadiene (which is conjugated) and 1,4 pentadiene (which is not conjugated). To recognize the differences between the two, let us look at the orbitals that are involved in the \(\pi\) system bonding. Recall that \(\pi\) bonds can be considered as the side-to-side overlap of p orbitals, so that the electron density lies above and below the plane of the rest of the molecule. Consider the orbitals in 1,3-pentadiene: there is overlap of \(\mathrm{p}\) orbitals that result in a continuous
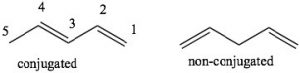
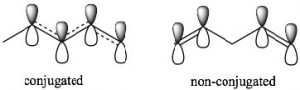
π orbital system over carbons 1-4. The consequence of this is that there is some partial double-bond character between carbons 2 and 3. In 1,4-pentadiene there is no possibility of overlap between the two separate \(\pi\) bonds. Note that in the non-conjugated system, there is an \(\mathrm{sp}^{3}\) hybridized carbon between the two sets of \(\mathrm{sp}^{2}\) hybridized carbon-carbon double bonds which prevents any overlap of (\(\mathrm{p}\)) orbitals on carbons 2 and 4.
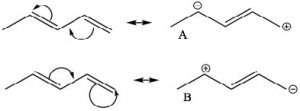
One way to indicate and predict where this partial double bond character can occur is to use resonance structures. We can write resonance structures for the conjugated system which have double bond character between \(\mathrm{C}-2\) and \(\mathrm{C}-3\). Note that since resonance contributors A and B are equivalent, there is no actual charge separation in this molecule. It is not possible (without breaking a sigma bond) to write resonance structures like this for 1,4-pentadiene (try and convince yourself that this is true ).
MolecularOrbitalTheory: Another model that can be used to describe the bonding is to consider the π system in terms of molecular orbital theory. Molecular orbital theory considers the bonding orbitals as extending over the whole molecule. The number of molecular orbitals is equal to the sum of all the atomic orbitals. In practice, this approach is far too complex for even the smallest of organic molecules, which is why we usually use the simpler valence bond model in which we consider bonds as being located between two atoms. In the case of conjugated systems, it is often helpful to use the valence bond approach for the sigma (single bonds) framework and then consider the conjugated π system using molecular orbital theory. In this case, if we have a conjugated system of two π bonds, then four p atomic orbitals are involved in forming the four molecular orbitals (\(\mathrm{MO}\)s). Recall that when molecular orbitals are formed from atomic orbitals (\(\mathrm{AO}\)s), if the (quantum mechanical) wave functions add in phase, the resulting energy of the \(\mathrm{MO}\) is lower—that is, the interaction is stabilizing and the \(\mathrm{MO}\)s are bonding \(\mathrm{MO}\)s. If the \(\mathrm{AO}\)s add out of phase, the interaction is destabilizing and the result is antibonding \(\mathrm{MO}\)s. Note that, in the diagram (\(\downarrow\)), only the lowest energy \(\mathrm{MO}\) has electron density between carbons 2 and 3. All the other \(\mathrm{MO}\)s have a node (no electron density) at this position. Overall, we see that there is more \(\pi\) electron density between \(\mathrm{C}-1\) and \(\mathrm{C}-2\), and between \(\mathrm{C}-3\) and \(\mathrm{C}-4\).
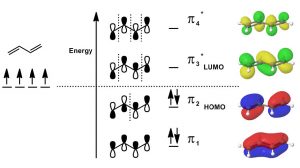
Since there are only 4 \(\pi\) electrons, only the two bonding \(\mathrm{MO}\)s are occupied while the two antibonding \(\mathrm{MO}\)s are unoccupied. As we will see, if we consider reactions using \(\mathrm{MO}\) theory, the Highest Occupied \(\mathrm{MO}\) (\(\mathrm{HOMO}\)) and the Lowest Unoccupied \(\mathrm{MO}\) (\(\mathrm{LUMO}\)) are the orbitals that participate in new bonding interactions. In general, we use the simplest model that allows us to predict and explain the outcome of reactions, which is usually valence bond theory, but we will call on \(\mathrm{MO}\) theory when necessary—and discussions of conjugated systems sometimes require \(\mathrm{MO}\) theory to explain phenomena.
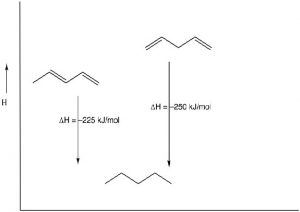
Stability of Conjugated Systems: As we have seen before, one way to identify the thermodynamic stability of alkenes is to reduce them to the corresponding alkane by adding \(\mathrm{H}_{2}\) (hydrogens) across the double bond and determine the enthalpy change.[1] In the case of 1,3- and 1,4-pentadiene, we can compare their heats of hydrogenation to produce the corresponding pentane; we find that the conjugated diene is about \(25 \mathrm{kJ/mol}\) more stable. This is a general finding: the more conjugated a system, the more stable it is and the less reactive it is.