6.3: Glycerolipids
- Page ID
- 432914
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Define glycerolipids and triglycerides and understand their structures.
- Understand the difference in composition and properties of fats and oils and their primary role as energy storage molecules.
- Understand the chemical properties of fats and oils, including hydrolysis, saponification, and partial or complete hydrogenation.
What is a glycerolipid?
Glycerolipids have two components: propane-1,2,3-triol, also called glycerol, and one, two, or three fatty acids. Recall that a condensation reaction between an alcohol and a carboxylic acid forms an ester by eliminating a water molecule. The following example shows the reaction of glycerol with three molecules of stearic acid, creating a triester.
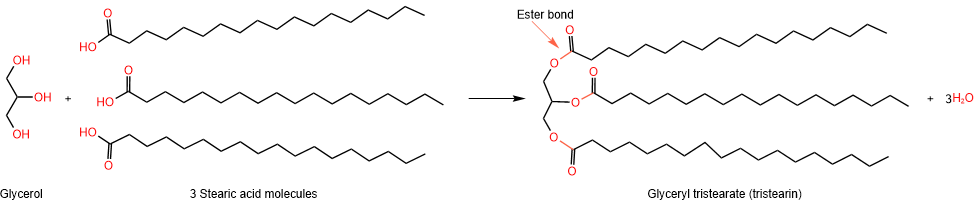
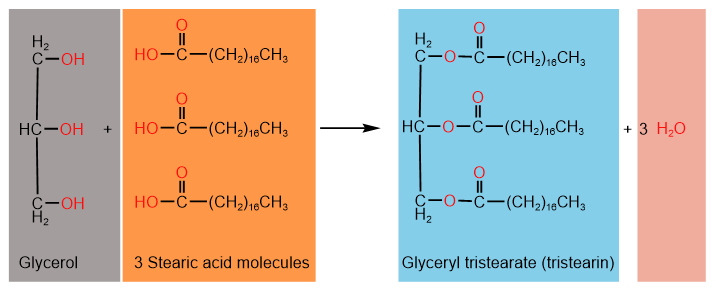
A mono-, di-, or tri-ester of glycerol is called glycerolipid.
The name of a glycerolipid begins with glyceryl and is followed by carboxylate. For example, the triester of glycerol shown above is glyceryl tristearate, which is commonly known as tristearin.
Glycerolipids that are triesters of glycerol with three fatty acids are called triacylglycerol or triglycerides. For example, glyceryl tristearate shown above is a triglyceride.
Triglycerides may be esters of three molecules of the same fatty acids, e.g., tristearin. Still, often the triglycerides found in nature are esters of two or three different fatty acids. Fatty acids usually found in fats and vegetable oils include lauric, myristic, palmitic, stearic, oleic, linoleic, and linolenic acids. An example of a mixed triglyceride of stearic, oleic, and palmitic acid is shown below.

Fats and oils
Fats and oils are triglycerides used as energy storage molecules in animals and plants.
 Energy storage is essential for hibernating animals that live in icy environments. They have plenty of food available during summer but no food and below-freezing temperatures in winter. They eat plants, seeds, and nuts and accumulate fat that becomes their energy source when hibernating in winter. For example, the polar bear shown on the right can accumulate up to 14 kg of fat per week in summer when the food is plenty and uses it as an energy source when the animal hibernates for 4 to 7 months in winter.
Energy storage is essential for hibernating animals that live in icy environments. They have plenty of food available during summer but no food and below-freezing temperatures in winter. They eat plants, seeds, and nuts and accumulate fat that becomes their energy source when hibernating in winter. For example, the polar bear shown on the right can accumulate up to 14 kg of fat per week in summer when the food is plenty and uses it as an energy source when the animal hibernates for 4 to 7 months in winter.
Although glycogen is a quick energy source, fats and oils are more energy dense for two reasons:
- first, fats and oils are in a more reduced form than glycogen and release more energy per mass upon oxidation, and
- second, glycogen is hydrophilic and absorbs water, increasing its mass.
Physical properties of fats and oils
Pure fats and oils are colorless, odorless, and tasteless. The color, taste, and odor associated with butter -an animal fat, and olive oil -a vegetable oil, is due to a small number of other substances mixed with the triglycerides.
Fats come from animal sources, such as meat, whole milk, lard, butter, and cheese. Vegetable oils come from plant sources, e.g., soybeans, peanuts, sunflowers, etc.
Fats are usually solid at room temperature because they have a higher proportion of long-chain unsaturated fatty acids with higher melting points, e.g., lard and butter shown in Figure \(\PageIndex{1}\). Vegetable oils are usually liquid at room temperature because they contain more unsaturated or short-chain saturated fatty acids.


For example, canola oil, safflower oil, flaxseed oil, sunflower oil, corn oil, olive oil, soyabean oil, peanut oil, and cottonseed oil are vegetable oils that are liquid at room temperature and have a higher proportion of unsaturated fatty acids as shown in Figure \(\PageIndex{2}\). Palm and coconut oils are vegetable oils with a higher balance of saturated fatty acids. Still, they are liquid at room temperature because they have short-chain saturated fatty acids. For example, coconut mainly comprises lauric acid (12:0), a short-chain fatty acid.


Chemical properties of fats and oils
Three important reactions of triglycerides are hydrolysis by water in the presence of acid or lipase enzyme, saponification, i.e., base-promoted hydrolysis, and hydrogenation of double bonds in the fatty acids.
Hydrolysis
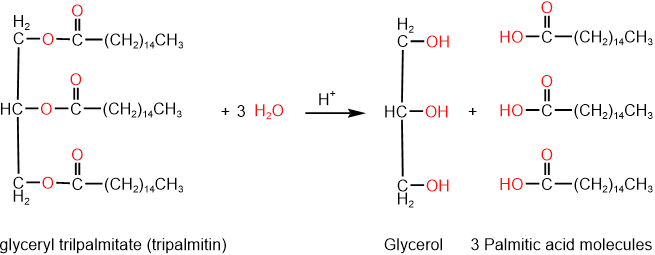
Easters are hydrolyzed (split) by water in the presence of an acid catalyst into alcohol and carboxylic acid. The same reaction happens with triglycerides, i.e., fats and vegetable oils—for example, tripalmitin hydrolyzes into glycerol and three palmitic acid molecules, as shown below.
Lipase enzymes do the same reaction during the digestion of triglycerides, e.g., trilaurin is hydrolyzed by lipase to glycerol and three molecules of lauric acid, as shown in the reaction below.
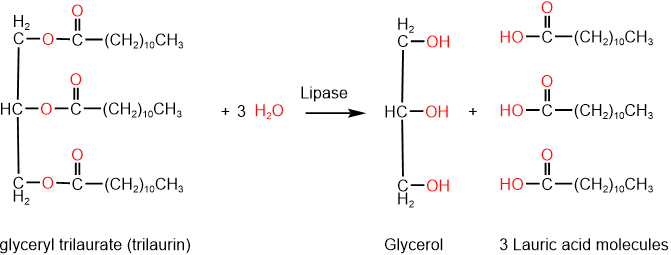
The digestion of lipids starts in the mouth with lingual lipases secreted by glands in the tongue and continues in the stomach with lingual and gastric lipases. Glycerol in the hydrolysis product is soluble in water, but fatty acids are not. Fatty acids are emulsified in the stomach and later mixed with bile and pancreatic juice to continue the process of digestion.
Saponification
Saponification is base-promoted hydrolysis of triglycerides that produces glycerol and salts of the fatty acids, a soap, as shown below for the case of saponification of tripalmitin.

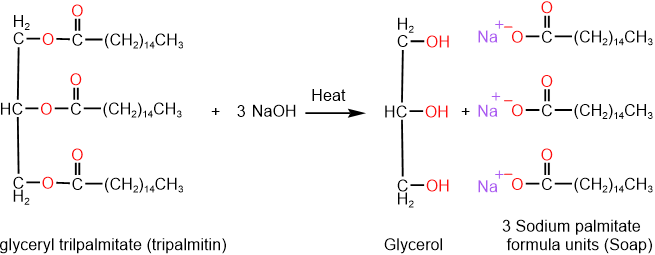
Different varieties of soaps are shown in the figure on the right. Sodium salts of fatty acids make hard soaps, while potassium salts make soft soaps. Three of the most common soaps are sodium stearate, oleate, and linoleate. Salts of saturated fatty acids make rigid, and those of polyunsaturated fatty acids make soft soap. Perfumes are added for scent and dyes for color, sand is added in scouring soap, and the air is blown into the soap to make it float on water.
Hydrogenation
Alkenes add \(\ce{H2}\) and covert to alkane in the presence of \(\ce{Ni}\), \(\ce{Pt}\), or \(\ce{Pd}\) catalyst. The same reaction happens with the \(\ce{C=C}\) in unsaturated fatty acids found in triglycerides. The conversion of unsaturated fatty acids to saturated fatty acids increases the melting point of triglycerides. Therefore, vegetable oils with more unsaturated fatty acids become semisolid or solid after partial or complete hydrogenation, as illustrated by the reaction in Figure \(\PageIndex{3}\). Hydrogenation of vegetable oils is a commercial process to convert vegetable oils to semisolid products like margarine and shortening.

The driving force for the industrial process of hydrogenation of vegetable oils is that margarine or shortenings are used as cheaper alternatives to butter with a longer shelf-life. Complete hydrogenation of vegetable oils converts them into hard margarine. Often partial hydrogenation is performed as the partially hydrogenated product is semisolid and mimics butter better than hard shortening. Dyes and flavors are mixed with it to mimic butter's color, taste, and odor.
Like all other processed food products, hydrogenated vegetable oil products are associated with health problems. Partial hydrogenation converts some of the cis-\(\ce{C=C}\) bonds to trans-\(\ce{C=C}\) bonds, as illustrated in Figure \(\PageIndex{3}\). Trans-\(\ce{C=C}\) bonds are rare in some fatty acids. Research reports indicate that trans-fatty acids affect blood cholesterol like unsaturated fatty acids. Research reports also indicated that trans-fatty acids increase the level of low-density lipids (LDL), considered bad cholesterol, and decrease the level of high-density lipids (HDL), regarded as good cholesterol. Health organizations force the manufacturers to show the trans-fatty acid contents on the product labels. They are trying to educate the consumers about trans-fatty acids, as shown in Figure \(\PageIndex{4}\) from U.S. Food and Drug Administration.



