6.4: Glycerophospholipids
- Page ID
- 432915
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Define glycerophospholipids and understand the structural features that allow them to organize into lipid bilayers.
- Understand the classification of glycerophospholipids and their role in infant respiratory distress syndrome.
What are glycerophospholipids?
Glycerophospholipids are triesters of propane-1,2,3-triol (glycerol): 1st and 2nd ester bonds with fatty acids, and the 3rd ester bond with phosphate, as illustrated in Figure \(\PageIndex{1}\). The phosphate group is often a diester: an ester with the primary alcohol of glycerol and 2nd ester bond with a small molecule, such as choline, ethanolamine, serine, or inositol.

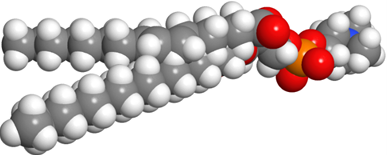
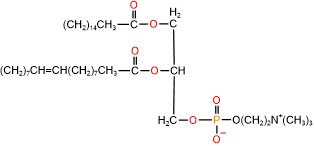
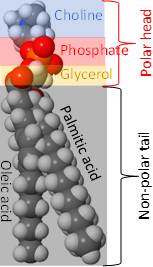 Phospholipids have two hydrophobic tails, which are the hydrocarbon groups of two fatty acids and a polar head comprising of ester groups with fatty acids, phosphate having a -ve charge, and often also have a +ve charge on the nitrogen of the small molecule attached with the phosphite, as shown in the figure on the right. The 1st fatty acid attached to the primary alcohol group is usually a saturated fatty acid, and the 2nd fatty acid attached to the secondary alcohol group of glycerol is usually an unsaturated fatty acid.
Phospholipids have two hydrophobic tails, which are the hydrocarbon groups of two fatty acids and a polar head comprising of ester groups with fatty acids, phosphate having a -ve charge, and often also have a +ve charge on the nitrogen of the small molecule attached with the phosphite, as shown in the figure on the right. The 1st fatty acid attached to the primary alcohol group is usually a saturated fatty acid, and the 2nd fatty acid attached to the secondary alcohol group of glycerol is usually an unsaturated fatty acid.
Classes of glycerophospholipids
The glycerol esterified with two fatty acids and one phosphoric acid is also called phosphatidyl. Phosphatidyls with phosphate esterified with choline are called phosphatidylcholines (lecithin). Phosphatidyl esterified with ethanolamine, or sometimes with serine, are called phosphatidylethanolamines or phosphatidylserine (common name cephalins). Another category is phosphatidylinositols with inositol-esterified phosphate, as shown in Figure \(\PageIndex{2}\).




Lipid bilayer
Recall the general solubility rule "like dissolves like". Oil droplets that are nonpolar floating on the surface of the water, which is polar, ultimately coalesce, making bigger droplets. Glycerophospholipids are amphiphilic, having an overall cylindrical shape with nonpolar tails of fatty acid chains and polar heads of ester groups on the glycerol part and ionic groups on phosphate and amine groups. Glycerophospholipids form a bilayer in the watery environment of living things based on the principle "like dissolves like", as illustrated in Figure \(\PageIndex{3}\).


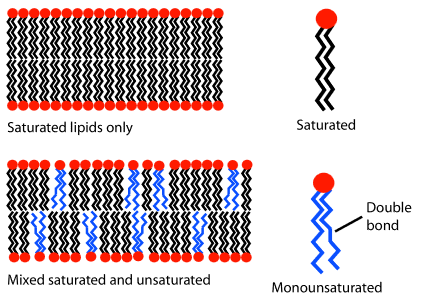
 The hydrophobic tails of fatty acids form the interior of the bilayer, and the hydrophilic polar heads make the outer layers in contact with water. The molecules are in a fluid (dynamic) state due to thermal energy (Figure \(\PageIndex{3}\) middle) but held together by intermolecular interactions: dipole-dipole interaction in the polar heads and London dispersion forces in the hydrophobic tails. Saturated fatty acids pack tightly together, making a rigid bilayer. However, adding unsaturated fatty acids with kinks makes the packaging lose, resulting in a more fluid bilayer, as illustrated in Figure \(\PageIndex{3}\) right.
The hydrophobic tails of fatty acids form the interior of the bilayer, and the hydrophilic polar heads make the outer layers in contact with water. The molecules are in a fluid (dynamic) state due to thermal energy (Figure \(\PageIndex{3}\) middle) but held together by intermolecular interactions: dipole-dipole interaction in the polar heads and London dispersion forces in the hydrophobic tails. Saturated fatty acids pack tightly together, making a rigid bilayer. However, adding unsaturated fatty acids with kinks makes the packaging lose, resulting in a more fluid bilayer, as illustrated in Figure \(\PageIndex{3}\) right.
The glycerophospholipid bilayer is a significant component of the cell membrane, the membranes around the nucleus, and some cell organelles. The hydrophobic nature of the interior of the bilayer becomes a barrier to the movement of ions and water in and out of the cell. Still, the bilayer's fluid nature allows the diffusion process to move some particles across the membrane. Other components of the cell membrane, such as cholesterol, adjust the rigidity, and proteins control the movement of particles in and out of the cell as needed. The structure of cell membranes is described in a later section.
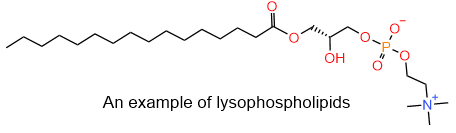 Snakes inject their venom through fangs when they bite their prey, as shown in the figure on the right. The venom of eastern diamondback rattlesnakes and Indian cobra contains phospholipase enzymes that catalyze the hydrolysis of fatty acid on the secondary hydroxyl group of glycerol. The resulting phospholipids with one less carboxylate group, called lysophospholipids, is no more cylindrical molecule and do not fit correctly in the lipid-bilayer, causing the red blood cell membrane to rupture. This poses a significant health risk and may kill humans. The structure of the lysophospholipid example is shown in the figure on the left.
Snakes inject their venom through fangs when they bite their prey, as shown in the figure on the right. The venom of eastern diamondback rattlesnakes and Indian cobra contains phospholipase enzymes that catalyze the hydrolysis of fatty acid on the secondary hydroxyl group of glycerol. The resulting phospholipids with one less carboxylate group, called lysophospholipids, is no more cylindrical molecule and do not fit correctly in the lipid-bilayer, causing the red blood cell membrane to rupture. This poses a significant health risk and may kill humans. The structure of the lysophospholipid example is shown in the figure on the left.
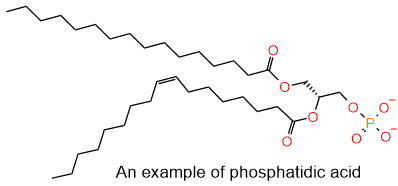 In the breathing process, the exchange of \(\ce{O2}\) and \(\ce{CO2}\) takes place in air sacs called alveoli within the lungs, as illustrated in Figure \(\PageIndex{4}\). Pulmonary surfactants, composed of a mixture of lecithin and sphingomyelin lipids, are released into the lungs of newborn babies that reduce the surface tension and ease the inflation of alveoli. Lecithins are mixtures of glycerophospholipids, including phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, phosphatidylserine (shown in Figure \(\PageIndex{2}\)) and phosphatidic acid shown in the figure on the right.
In the breathing process, the exchange of \(\ce{O2}\) and \(\ce{CO2}\) takes place in air sacs called alveoli within the lungs, as illustrated in Figure \(\PageIndex{4}\). Pulmonary surfactants, composed of a mixture of lecithin and sphingomyelin lipids, are released into the lungs of newborn babies that reduce the surface tension and ease the inflation of alveoli. Lecithins are mixtures of glycerophospholipids, including phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, phosphatidylserine (shown in Figure \(\PageIndex{2}\)) and phosphatidic acid shown in the figure on the right.
In the cases of premature birth, before 28 weeks of gestation, the level of the surfactant is insufficient, causing the air sacs to collapse and have to reopen with each breathing, a condition called infant respiratory distress syndrome. This makes breathing difficult, may damage the alveoli, and lead to hypoxia and acidosis due to less oxygen inhalation and less carbon dioxide exhalation.
The lecithin to sphingomyelin ratio in mature fetal lungs is ~2.5, the ratio 2.4 to 1.6 poses a low risk, and less than 1.5 poses a high risk of infant respiratory distress syndrome. Treatment includes steroids given to the mother or infant to assist the development of lungs, the application of surfactants, and the use of a ventilator to help breathing.



