4.3: Acid-base reactions
- Page ID
- 419537
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Recognize acids and bases, understand the strength of acids and based, and predict the strength based on the structural features of the molecules.
- Understand simple acid-base reactions that are elementary steps in organic reaction mechanisms.
- Predict the direction of acid-base equilibrium based on the acid/base strength.
- Predict the ionization of acid/base functional groups in biochemicals under physiological conditions.
What is an acid-base reaction?
An acid-base reaction is a reaction in which a proton (\(\ce{H^{+}}\)) is exchanged between reactants. For example, when acetic acid (\(\ce{CH3COOH}\)) is mixed with water, a proton is transferred from acetic acid to water, as shown in the reaction equation below.
\(\ce{CH3COOH + H2O <=> CH3COO^{-} + H3O^{+}}\)
An acid donates a proton, and a base accepts a proton. \(\ce{CH3COOH}\) is an acid and \(\ce{H2O}\) is a base in the forward reaction. \(\ce{CH3COO^{-}}\) is a base and \(\ce{H3O^{+}}\) is an acid in the reverse reaction. Two species that are related by the addition or removal of a proton are conjugate acid-base pairs. For example, \(\ce{CH3COOH}\) and \(\ce{CH3COO^{-}}\) are conjugate acid-base pair. Similarly, \(\ce{H3O^{+}}\) and \(\ce{H2O}\) are conjugate acid-base pair. Since a proton has a +1 charge, its removal from an acid decreases the charge by one, and its addition to a base increases the charge by one. So, a general formula of an acid-conjugate acid-base pair could be represented as \(\ce{HB}\) and \(\ce{B^{-}}\), e.g., \(\ce{CH3COOH}\) and \(\ce{CH3COO^{-}}\) or as \(\ce{HB^{+}}\) and \(\ce{B}\), e.g., \(\ce{H3O^{+}}\) and \(\ce{H2O}\). Acid-base reactions are generally fast and reversible, establishing equilibrium. That is why equilibrium arrows are shown separating reactants and products.
Strength of an acid and a base
Strength of an acid
The strength of an acid is a measure of its ability to donate a proton to a base. Often the solvent is water, and the reference base is \(\ce{H2O}\), as in the following general reaction.
\[\ce{HA + H2O <=> A^{-} + H3O^{+}}\nonumber \]
The relationship of molar concentrations of reactants and products in an equilibrium reaction is expressed by the following formula:
\[\ce{K_{eq} = \frac{[A^{-}][H3O^{+}]}{[HA][H2O]}}\nonumber \]
,where square brackets indicate the molar concentration of the specie within the brackets and \(\ce{K_{eq}}\) is a constant called the equilibrium constant. Since water is a solvent, its concentration \(\ce{[H2O]}\) is almost a constant and merged with the constant to define a new constant, as shown below.
\[\ce{ K_{a} = K_{eq}[H2O] = \frac{[A^{-}][H3O^{+}]}{[HA]}}\nonumber \]
, where \(\ce{K_{a}}\) is a constant called acid dissociation constant that expresses the strength of the acid, the higher the value of \(\ce{K_{a}}\); the stronger the acid. Since \(\ce{K_{a}}\) is often a large negative number, it is expressed on a log scale with its signed reversed, as shown in the following formula.
\[\ce{pK_{a} = -Log_{10}(K_{a})}\nonumber \]
The \(\ce{pK_{a}}\) is a quantitative measure of the acid strength, i.e., the smaller the \(\ce{pK_{a}}\) means the large the \(\ce{K_{a}}\) and stronger the acid.
Strength of a base
The strength of a base is a measure of its ability to accept a proton from an acid. Often the solvent is water, and the reference base is
\(\ce{H2O}\), as in the following general reaction.
\[\ce{A^{-} + H2O <=> HA+ HO^{-}}\nonumber \]
The relationship between molar concentrations of reactants and products in an equilibrium reaction is expressed by the following formula:
\[\ce{K_{eq} = \frac{[HA][HO^{-}]}{[A^{-}][H2O]}}\nonumber \]
Again \(\ce{[H2O]}\) is assumed to be a constant and merged with the constant to define a new constant, as shown below.
\[\ce{ K_{b} = K_{eq}[H2O] = \frac{[HA][HO^{-}]}{[A^{-}]}}\nonumber \]
, where \(\ce{K_{b}}\) is a constant called base dissociation constant that expresses the strength of the base. the higher the value of \(\ce{K_{b}}\) the stronger the base. again take a log and reverse the sign to arrive at a constant called \(\ce{pK_{b}}\) as shown in the following formula.
\[\ce{pK_{b} = -Log_{10}(K_{b})}\nonumber \]
The \(\ce{pK_{b}}\) is a quantitative measure of the base strength, i.e., the smaller the \(\ce{pK_{b}}\) means the large the \(\ce{K_{b}}\) and stronger the base.
Relationship between \(\ce{pK_{a}}\) and \(\ce{pK_{b}}\) of conjugate acid-base pair
The \(\ce{K_{a}}\) of an acid \(\ce{HA}\) and \(\ce{K_{b}}\) of a conjugate acid-base \(\ce{A^{-}}\) pair are reciprocal of each other as proven below.
\[\ce{ {K_{a}} \times {K_{b}} = \frac{[A^{-}][H3O^{+}]}{[HA]}\times \frac{[HA][HO^{-}]}{[A^{-}]} = {[HO^{-}][H3O^{+}]} = K_{w} = 10^{-14}}\nonumber \]
That rearranges to,
\[\ce{ {K_{b}} = \frac{10^{-14}}{K_{a}}}\nonumber \]
It shows that if acid is a strong acid (large \(\ce{K_{a}}\)), its conjugate base is a weak base ((small \(\ce{K_{b}}\)), and vice versa. Taking the log and changing the sign on both sides leads to a relationship between \(\ce{pK_{a}}\) and \(\ce{pK_{b}}\) of conjugate acid-base pair, as shown below.
\[\ce{ -Log_{10}{K_{b}} = -Log_{10}(\frac{10^{-14}}{K_{a}})}\nonumber \]
\[\ce{ pK_{b} = 14 - pK_{a}}\nonumber \]
Therefore, \(\ce{ pK_{a}}\) values are tabulated in the reference books, and \(\ce{ pK_{b}}\) values, if needed, are calculated from the \(\ce{ pK_{a}}\) values of their conjugate acids using the above formula.
Factors affecting the strength of acids
The \(\ce{pK_{a}}\) is a quantitative measure of acid strength, i.e., the smaller the \(\ce{pK_{a}}\), the stronger the acid. For example, acetic acid (\(\ce{CH3COOH, pK_{a} = 4.76}\)) is a stronger acid than ethanol (\(\ce{CH3CH2OH, pK_{a} = 15.9}\)). Approximate \(\ce{pK_{a}}\) of acids commonly encountered in organic chemistry are listed in Table 1.
| \(\ce{pK_a}\): | <0 | 2 | 5 | 10 | 15 |
| Compounds: |
Strong acids like sulfuric acid \(\ce{HO-\!\!\!\!{\overset{\overset{\huge\enspace{O}}|\!\!|\enspace}{\underset{\underset{\huge\enspace\!{O}}|\!\!|\enspace}{S\!}}}\!\!\!-OH}\) |
Phosphate esters \(\ce{RO-\!\!\!\!\!{\overset{\overset{\huge\enspace{O}}|\!\!|\enspace}{\underset{\underset{\huge\enspace\,{OH}} |}{P}}}\!\!\!\!-OH}\), \(\ce{RO-\!\!\!\!\!{\overset{\overset{\huge\enspace{O}}|\!\!|\enspace}{\underset{\underset{\huge\enspace\,{OR}} |}{P}}}\!\!\!\!-OH}\) |
Carboxylic acids \(\ce{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-OH}\) Phosphate esters \(\ce{RO-\!\!\!\!\!{\overset{\overset{\huge\enspace{O}}|\!\!|\enspace}{\underset{\underset{\huge\enspace\,{OH}} |}{P}}}\!\!\!\!-O^{-}}\) |
Phenols Ammonium ions \(\ce{R-NH3^{+}}\) Thiols \(\ce{R-SH}\) |
Water \(\ce{H2O}\) Alcohols \(\ce{R-OH}\) |
| \(\ce{pK_a}\): | 20 | 25 | 35 | 45 | >50 |
| Compounds: | Aldehydes and ketones \(\ce{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-CH2-R}\) | Terminal alkynes \(\ce{R-C≡CH}\) |
Hydrogen \(\ce{H-H}\) Amines \(\ce{R-NHR}\) |
Alkenes \(\ce{R2C=CHR}\) | Alkanes \(\ce{R-CH2-R}\) |
The major factors affecting the acid strength are the following.
Electronegativity
Within the same row of the periodic table, the more electronegative the atom to which the proton is bonded, the more acidic the proton is. Electronegativity (EN) increases from left to right in the same row, e.g., \(\ce{C}\), EN 2.6 < \(\ce{N}\), EN 3.0 < \(\ce{O}\), EN 3.4 < \(\ce{F}\), EN 4.0.
The acid strength increases as the electronegativity of the atom carrying acidic proton increases within the same row of the periodic table, e.g., \(\ce{CH4, pK_{a}}\) ~60 < \(\ce{NH3, pK_{a}}\) ~38 < \(\ce{H2O, pK_{a}}\) 14.0 < \(\ce{HF, pK_{a}}\) 3.2.
This effect of electronegativity explains why alcohols (\(\ce{R-OH}, pK_a \text ~16\)) are more acidic than amines (\(\ce{R-NHR}, pK_a \text ~40\)) and amines are more acidic than alkanes (\(\ce{R-CH3}, pK_a \text ~60\)). This trend related to electronegativity does not hold when comparing elements from different rows.
Size of atom
The electronegativity decreases from top to bottom of a periodic table, e.g., for halogens: \(\ce{F}\), EN 3.98 > \(\ce{Cl}\), EN 3.16 > \(\ce{Br}\), EN 2.69 > \(\ce{I}\), 2.66. . The size of atoms has an opposite trend, i.e., the size increases from top to bottom of the periodic table, e.g., the size of halogens varies in this order: \(\ce{F}\) < \(\ce{Cl}\) < \(\ce{Br}\) < \(\ce{I}\).
The acid strength increases as the size of the atom carrying the acidic proton increases in the same period, e.g., \(\ce{HF, pK_{a}}\) 3.2 < \(\ce{HCl, pK_{a}}\) -7 < \(\ce{HBr, pK_{a}}\) -8 < \(\ce{HI, pK_{a}}\) -9.9.
This is because as the size of the atom increases, its bond with a proton becomes weaker and easier to break. Further, the negative charge a proton leaves behind is more easily stabilized when spread over a larger atom than a smaller atom. The effect of the size of an atom on acid strength is opposite to that of electronegativity within the same period.
The effect of the size of the atom bearing acidic proton on acidity is dominant over the effect of electronegativity.
The effect of size explains why thiols (\(\ce{R-SH}, pK_a \text ~10\)) are stronger acids than alcohols (\(\ce{R-OH}, pK_a \text ~16\)).
Resonance
When a proton leaves an acid \(\ce{HA}\), it usually leaves a negative charge on the conjugate base \(\ce{A^{-}}\). Resonance can stabilize the negative charge by distributing it on more than one atom.
An acid with conjugated base resonance stability can let go of its acidic proton more easily. It is stronger than an equivalent acid with no resonance stabilization of its conjugate base.
For example, both carboxylic acids and alcohols have acidic proton on an \(\ce{-OH}\) group, but carboxylic acids are stronger acids (\(\ce{pK_{a}, \text ~5}\)) than alcohols (\(\ce{pK_{a}, \text ~16}\)) due to resonance stabilization of the negative charge on their conjugate bases, as illustrated below.
Carboxylic acid: 
Alcohol: 
Phenols are also stronger acids (\(\ce{pK_{a}, \text ~10}\)) than alcohols for the same reason, as shown below.

However, phenols are weaker acids than carboxylic acids because the negative charge is shared by more electronegative \(\ce{O}\) in carboxylic acids than by less electronegative \(\ce{C}\) in phenols.
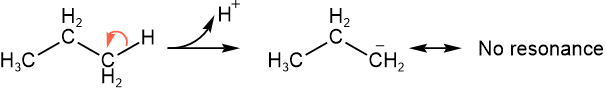
Protons on \(\ce{\alpha C}\) to a \(\ce{C=O}\) group, as in aldehydes, ketones, and carboxylic acid derivatives, are more acidic (\(\ce{pK_{a}, \text ~20}\)) than protons on alkanes (\(\ce{pK_{a}, \text ~60}\)) due to the resonance effect, as shown below.
Hybridization
Electrons in an s-orbital are nearer to and more attracted by the nucleus than in a p-orbital of the same shell. This is because of the spherical shape of the s-orbital placing electrons nearer to the nucleus versus the dumbbell shape of the p-orbital in the same shell. Therefore, the valence electrons in an sp-orbital having 50% s-character are attracted more to the nucleus than an sp2 orbital with 33% s-character and an sp3 orbital with 25% s-character. Recall that electronegativity is a measure of the ability of a nucleus to attract valence electrons.
The electronegativity of an atom and, consequently, the acidity of a proton attached to it increase with a change in hybridization in this order:sp3 < sp2 < sp.
For example, the acidity of protons of sp3-hybridized ethane, sp2-hybridized ethene, and sp-hybridized ethyne increases in this order: \(\ce{CH3CH3}\) \(pK_a \;51\) < \(\ce{CH2=CH2}\) \(pK_a \;44\) < \(\ce{CH≡CH}\) \(pK_a \;25\).
Inductive effect
The electron-withdrawing effect of an electronegative atom, i.e., the inductive effect increases the acidity of the proton adjacent to it.
- Near the electronegative atom stronger, the effect, as illustrated in the example below where \(\ce{Cl}\) is the electronegative atom affecting the acidity of \(\ce{-COOH}\) proton.

- The more electronegative the atom stronger the effect, as illustrated in the example below, where the electronegativity increases in this order: \(\overrightarrow{\ce{ H < I < Br < Cl < F}}\).
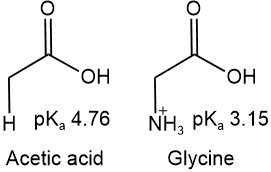
Amino acids are more acidic than carboxylic acids due to the inductive effect of ammonium ion, e.g., glycine is more acidic than acetic acid as shown below.
Charge
A proton \(\ce{H^{+}}\) on a positively charged specie is easier to remove and, consequently, more acidic than on the same species in a neutral state.
The acidity of the following species reflects this: \(\overrightarrow{\ce{H2O, pK_a \;\text15.7 < H3O^{+}, pK_a \;\text-1.74}}\) and \(\overrightarrow{\ce{NH3, pK_a \;\text ~38 < NH4^{+}, pK_a \;\text 9.24}}\). The effect of the charge is reduced but still exists when the charge is on an atom other than the atom bearing the acidic proton. Negatively charges species, conversely, are less acidic than the same specie in a neutral or positively charged state, as illustrated below for the case of phosphoric acid species.
\(\ce{HO-\!\!\!\!\!{\overset{\overset{\huge\enspace{O}}|\!\!|\enspace}{\underset{\underset{\huge\enspace\,{OH}} |}{P}}}\!\!\!\!-OH}\), \(\ce{pK_a \;\text 2.14}\) > \(\ce{^{-}O-\!\!\!\!\!{\overset{\overset{\huge\enspace{O}}|\!\!|\enspace}{\underset{\underset{\huge\enspace\,{OH}} |}{P}}}\!\!\!\!-OH}\), \(\ce{pK_a \;\text 7.20}\) > \(\ce{^{-}O-\!\!\!\!\!{\overset{\overset{\huge\enspace{O}}|\!\!|\enspace}{\underset{\underset{\huge\enspace\,{O^{-}}} |}{P}}}\!\!\!\!-OH}\), \(\ce{pK_a \;\text 12.37}\)
Examples of organic acid-base reactions
Reactions of organic acids
Carboxylic acids are organic acids. The following example shows that when a carboxylic acid is dissolved in water, it dissociates by donating its proton to water.
\(\ce{CH3COOH + H2O <=> CH3COO^{-} + H3O^{+}}\)
The reaction is written as an equilibrium because it goes in both directions, forward and reverse. acetic acid \(\ce{CH3COOH}\) is the acid in reactants and hydronium ion \(\ce{H3O^{+}}\) is the acid in products.
The general rule is that a stronger acid goes to a weaker one in an acid-base reaction. It is equally correct to say a stronger base goes to a weaker base.
For example, it is more accurate to say that the above reaction is reverse directed, or there is more concentration of reactants than the products at equilibrium, because \(\ce{H3O^{+}}\) is a stronger acid than \(\ce{CH3COOH}\). This fact is represented by a longer equilibrium arrow in the direction of weaker acid in the equilibrium, as shown below.
\[\ce{\underset{pK_a \;\text 4.76}{CH3COOH} + H2O <<=> CH3COO^{-} + \underset{pK_a \;\text -1.74}{H3O^{+}}}\nonumber \]
This reaction is made strongly forward directed by using a strong base like \(\ce{^{-}OH}\) that produces a weaker acid \(\ce{H2O}\), making the reaction almost irreversible, as shown below.
\[\ce{\underset{pK_a \;\text 4.76}{CH3COOH} + ^{-}OH -> CH3COO^{-} + \underset{pK_a \;\text 14.0}{H2O}}\nonumber \]
This is an example of an acid-base neutralization reaction. The \(\ce{^{-}OH}\) ion is obtained by dissolving sodium hydroxide \(\ce{NaOH}\) in water, but \(\ce{Na^{+}}\) is a spectator ion, i.e., it does not take part in the reaction and is usually not shown in the equation. As shown below, other organic acids, like phenols and thiols, do similar acid-base neutralization reactions.
\[\ce{\underset{pK_a \;\text 9.95}{C6H5OH} + ^{-}OH -> C6H5O^{-} + \underset{pK_a \;\text 14.0}{H2O}}\nonumber \]
\[\ce{\underset{pK_a \;\text 10.4}{CH3SH} + ^{-}OH -> CH3S^{-} + \underset{pK_a \;\text 15.7}{H2O}}\nonumber \]
Terminal alkyne has acidic protons but they are weak acids (\(\ce{pK_a \;\text ~25}\)). A base like \(\ce{^{-}OH}\) is not a strong enough base to remove all of the alkyne protons, as shown by the following equilibrium reaction.
\[\ce{\underset{pK_a \;\text 25}{R-C≡CH} + ^{-}OH <<=> R-C≡C^{-} + \underset{pK_a \;\text 14.0}{H2O}}\nonumber \]
An amide anion (\(\ce{^{-}NH2}\) in the form of \(\ce{NaNH2}\)) is a sufficiently strong base to neutralize alkyne, as shown below.
\[\ce{\underset{pK_a \;\text 25}{R-C≡CH} + ^{-}NH2 -> R-C≡C^{-} + \underset{pK_a \;\text 38}{NH3}}\nonumber \]
Alcohols (\(\ce{R-OH, pK_a \;\text ~25}\)) are also weak acids and not fully neutralized with bases like \(\ce{^{-}OH}\), as shown below.
\[\ce{\underset{pK_a \;\text 16}{R-OH} + ^{-}OH <=> R-O^{-} + \underset{pK_a \;\text 14}{H2O}}\nonumber \]
A hydride ion (\(\ce{H^{-}}\) in the form of \(\ce{NaH}\)) is a sufficiently strong base to neutralize alcohols, as shown below.
\[\ce{\underset{pK_a \;\text 16}{R-OH} + H^{-} -> R-O^{-} + \underset{pK_a \;\text 35}{H2}}\nonumber \]
Reactions of organic bases
Amines (\(\ce{R-NH2}\)) are organic bases that produce \(\ce{^{-}OH}\) ions in water, as shown below.
\[\ce{\underset{pK_a \;\text 15.7}{H2O} + R-NH2 <<=> ^{-}{OH} + \underset{pK_a \;\text 10}{R-NH3^{+}}}\nonumber \]
Amines neutralize strange acids like (\(\ce{HCl}\)) and produce ammonium salts, as shown below.
\[\ce{\underset{pK_a \;\text -7}{HCl} + R-NH2 -> R-NH3^{+}{Cl^{-}}}\nonumber \]
Many drugs contain amine functional groups. Amines are usually unstable and insoluble in water. Neutralizing the amine (\(\ce{R-NH2}\)) with \(\ce{HCl}\) converts them to ammonium chloride salts (\(\ce{R-NH3^{+}C^{-}}\)), also called amine.HCl salts (\(\ce{R-NH2.HCl}\)), which are water-soluble and more stable. Therefore, drugs containing amine functional groups are usually sold in ammonium chloride form, making them soluble in body fluids like blood plasma and increasing their shelf-life. For example, methadone, a narcotic analgesic, and procaine, a local anesthetic, are marketed as hydrochloride salts, as shown below.
 Methadone.\(\ce{HCl}\)
Methadone.\(\ce{HCl}\)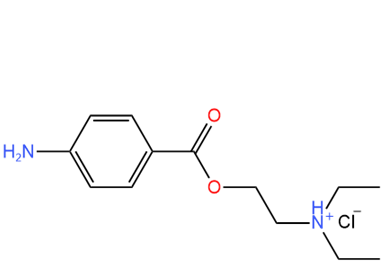 Procaine.\(\ce{HCl}\) (also called novocain)
Procaine.\(\ce{HCl}\) (also called novocain)Alcohols are amphoteric substances like water, i.e., they can donate a proton as acid and accept a proton as bases. Like alcohols, the oxygen of carbonyl (\(\ce{C=O}\) group also has two lone pairs and can accept a proton. A strong acid like \(\ce{HCl}\) or \(\ce{H2SO4}\)can protonate an alcohol or a carbonyl group, as shown below.
\[\ce{\underset{pK_a \;\text -7}{HCl} + R-OH <=>> Cl^{-} + \underset{pK_a \;\text ~-3}{R-OH2^{+}}}\nonumber \]
\[\ce{\underset{pK_a \;\text -9}{H2SO4} + R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-R <=>> Cl^{-} + \underset{pK_a \;\text ~-7}{R-\!\!\!\!\!\!{\overset{\overset{\huge\enspace\enspace{\overset{\Large{+}}{O}H}}|\!\!\!\!\!\!\!|\enspace\enspace}{C}}\!\!\!\!\!-R}}\nonumber \]
The acid-base reactions that produce cations by accepting protons, like \(\ce{R-OH2^{+}}\) or \(\ce{R-\!\!\!\!\!\!{\overset{\overset{\huge\enspace\enspace{\overset{\Large{+}}{O}H}}|\!\!\!\!\!\!\!|\enspace\enspace}{C}}\!\!\!\!\!-R}\) and anions by donating protons, like \(\ce{R-O^{-}}\), \(\ce{R-C≡C^{-}}\), \(\ce{R-S^{-}}\), etc. are important intermediates in organic reactions described in the later sections.
Ionization of acidic and basic functional groups at physiological pH
The Henderson-Hasselbach equation for an acid (\(\ce{HA}\)) is: \[\color{blue}\text {Henderson-Hasselbach equation: } \ce{ pH = pK_{a} + Log_{10}(\frac{[A^{-}]}{[HA]})}\nonumber \], where \(\ce{pH = -Log_{10}[H3O^{+}]}\), and \(\ce{pK_{a} = -Log_{10}K_{a}}\). For a base (\(\ce{B}\)) is: \[\color{blue}\ce{ pH = pK_{a} + Log_{10}(\frac{[HB^{+}]}{[B]})}\nonumber \], where \(\ce{pK_a}\) is that of the conjugate acid \(\ce{HB^{+}}\). The Henderson-Hasselbach equation helps estimate the ratio of acid (\(\ce{HA}\)) to its conjugate base ((\(\ce{A^{-}}\)) and of base (\(\ce{HB}\)) to its conjugate acid ((\(\ce{B^{+}}\)) at the pH of the medium. According to Henderson-Hasselbach equaiton,
- for an acid (\(\ce{HA}\)), if \(\ce{pH}\) of medium is two units lower than the \(\ce{pK_a}\) of the acid, it will be present substantially in its de-protonated form \(\ce{A^{-}}\), and
- for a base (\(\ce{B}\)), if \(\ce{pH}\) of medium is two units higher than the \(\ce{pK_a}\) of its conjugated acid \(\ce{HB^{+}}\), it will be present substantially in its protonated form \(\ce{HBA^{+}}\).
Following conclusions are drawn by applying the above rules to the functional groups commonly found in cells.
- The \(\ce{pH}\) of cells is generally between 7 and 8.5. Carboxylic acids (\(\ce{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-OH}\)) have \(\ce{pK_a}\) 4 to 5. So, carboxylic acids exist in an ionized form \(\ce{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-O^{-}}\) in a physiological medium.
- The conjugate acid forms of amines \(\ce{R-NH3^{+}}\) have \(\ce{pK_{a}}\) 9 to 11. So, amines exist in the ionized form \(\ce{R-NH3^{+}}\) in a physiological medium.
- Amino acids \(\ce{R-\!\!\!\!\!\!\!\!\!{\underset{\underset{\huge\enspace\enspace\enspace{NH2}} |}{C}}\!\!\!\!\!\!\!\!\!H-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-OH}\) have amine and carboxylic acid groups. Amino acids exist as \(\ce{R-\!\!\!\!\!\!\!\!\!{\underset{\underset{\huge\enspace\enspace\enspace\underset{\small{+}}{N}{H3}} |}{C}}\!\!\!\!\!\!\!\!\!H-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-O^{-}}\). A specie with separate positive and negative charge groups on it is called a zwitterion. Amino acids exist as a zwitterions.
- Phosphodiesters \(\ce{RO-\!\!\!\!\!{\overset{\overset{\huge\enspace{O}}|\!\!|\enspace}{\underset{\underset{\huge\enspace\,{OR}} |}{P}}}\!\!\!\!-OH}\) present in DNA and RNA molecules have \(\ce{pK_{a}}\) 1 to 3. So, phosphodiester also exist in an ionized form \(\ce{RO-\!\!\!\!\!{\overset{\overset{\huge\enspace{O}}|\!\!|\enspace}{\underset{\underset{\huge\enspace\,{OR}} |}{P}}}\!\!\!\!-O^{-}}\) in a physiological medium.
- The conjugate acid forms of amines, i.e., ammonium ion \(\ce{R-NH3^{+}}\) have \(\ce{pK_{a}}\) 9 to 11. So, amines exist as ammonium ions \(\ce{R-NH3^{+}}\) in a physiological medium.
The equation is derived by performing the following steps: i) re-arranging \(\ce{ K_{a}}\) expression:
\[\ce{ K_{a} = \frac{[A^{-}][H3O^{+}]}{[HA]}}\nonumber \]
\[\ce{ [H3O^{+}] = K_{a}\times\frac{[HA]}{[A^{-}]}}\nonumber \]
Taking the log of both sides and reversing the sign:
\[\ce{ -Log_{10}[H3O^{+}] = -Log_{10}(K_{a}\times\frac{[HA]}{[A^{-}]})}\nonumber \]
\[\ce{ -Log_{10}[H3O^{+}] = -Log_{10}K_{a}-Log_{10}(\frac{[HA]}{[A^{-}]})}\nonumber \]
making a substitution for \(\ce{ pK_{a}}\), and \(\ce{ pH}\):
\[\boxed{\text {Henderson-Hasselbach equation: } \ce{ pH = pK_{a} + Log_{10}(\frac{[A^{-}]}{[HA]})}}\nonumber \]
, where \(\ce{pH = -Log_{10}[H3O^{+}]}\), and \(\ce{pK_{a} = -Log_{10}K_{a}}\).
A similar derivation of a general base \(\ce{B}\) and its conjugate acid \(\ce{HB^{+}}\) leads to this equation:
\[\boxed{\ce{ pH = pK_{a} + Log_{10}(\frac{[HB^{+}]}{[B]})}}\nonumber \]
, where \(\ce{pK_a}\) is that of the conjugate acid \(\ce{HB^{+}}\).